Human olfactory mucosa multipotent mesenchymal stromal cells promote survival, proliferation, and differentiation of human hematopoietic cells
- PMID: 22471939
- PMCID: PMC3495125
- DOI: 10.1089/scd.2012.0084
Human olfactory mucosa multipotent mesenchymal stromal cells promote survival, proliferation, and differentiation of human hematopoietic cells
Abstract
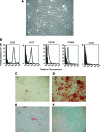
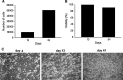
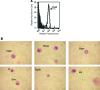
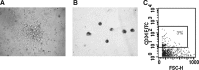
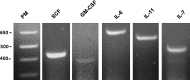
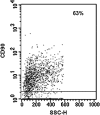
Multipotent mesenchymal stromal cells (MSCs) from the human olfactory mucosa (OM) are cells that have been proposed as a niche for neural progenitors. OM-MSCs share phenotypic and functional properties with bone marrow (BM) MSCs, which constitute fundamental components of the hematopoietic niche. In this work, we investigated whether human OM-MSCs may promote the survival, proliferation, and differentiation of human hematopoietic stem cells (HSCs). For this purpose, human bone marrow cells (BMCs) were co-cultured with OM-MSCs in the absence of exogenous cytokines. At different intervals, nonadherent cells (NACs) were harvested from BMC/OM-MSC co-cultures, and examined for the expression of blood cell markers by flow cytometry. OM-MSCs supported the survival (cell viability >90%) and proliferation of BMCs, after 54 days of co-culture. At 20 days of co-culture, flow cytometric and microscopic analyses showed a high percentage (73%) of cells expressing the pan-leukocyte marker CD45, and the presence of cells of myeloid origin, including polymorphonuclear leukocytes, monocytes, basophils, eosinophils, erythroid cells, and megakaryocytes. Likewise, T (CD3), B (CD19), and NK (CD56/CD16) cells were detected in the NAC fraction. Colony-forming unit-granulocyte/macrophage (CFU-GM) progenitors and CD34(+) cells were found, at 43 days of co-culture. Reverse transcriptase-polymerase chain reaction (RT-PCR) studies showed that OM-MSCs constitutively express early and late-acting hematopoietic cytokines (i.e., stem cell factor [SCF] and granulocyte- macrophage colony-stimulating factor [GM-CSF]). These results constitute the first evidence that OM-MSCs may provide an in vitro microenvironment for HSCs. The capacity of OM-MSCs to support the survival and differentiation of HSCs may be related with the capacity of OM-MSCs to produce hematopoietic cytokines.
Figures

Similar articles
-
Simultaneous expansion and harvest of hematopoietic stem cells and mesenchymal stem cells derived from umbilical cord blood.J Mater Sci Mater Med. 2010 Dec;21(12):3183-93. doi: 10.1007/s10856-010-4167-5. Epub 2010 Oct 6. J Mater Sci Mater Med. 2010. PMID: 20924776
-
Toll-like receptors 2 and 4 mediate the capacity of mesenchymal stromal cells to support the proliferation and differentiation of CD34⁺ cells.Exp Cell Res. 2012 Feb 1;318(3):196-206. doi: 10.1016/j.yexcr.2011.11.001. Epub 2011 Nov 10. Exp Cell Res. 2012. PMID: 22100911
-
17β-estradiol induces the proliferation of hematopoietic stem cells by promoting the osteogenic differentiation of mesenchymal stem cells.Tohoku J Exp Med. 2014 Jun;233(2):141-8. doi: 10.1620/tjem.233.141. Tohoku J Exp Med. 2014. PMID: 24898803
-
Influential factors for optimizing and strengthening mesenchymal stem cells and hematopoietic stem cells co-culture.Mol Biol Rep. 2024 Jan 25;51(1):189. doi: 10.1007/s11033-023-09041-9. Mol Biol Rep. 2024. PMID: 38270694 Review.
-
Is CD34 truly a negative marker for mesenchymal stromal cells?Cytotherapy. 2012 Nov;14(10):1159-63. doi: 10.3109/14653249.2012.729817. Cytotherapy. 2012. PMID: 23066784 Free PMC article. Review.
Cited by
-
Differential marker expression by cultures rich in mesenchymal stem cells.BMC Cell Biol. 2013 Dec 5;14:54. doi: 10.1186/1471-2121-14-54. BMC Cell Biol. 2013. PMID: 24304471 Free PMC article.
-
Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases.Front Immunol. 2021 Feb 5;11:591065. doi: 10.3389/fimmu.2020.591065. eCollection 2020. Front Immunol. 2021. PMID: 33613514 Free PMC article. Review.
-
Cell-based therapy for prevention and reversal of myocardial remodeling.Am J Physiol Heart Circ Physiol. 2012 Aug 1;303(3):H256-70. doi: 10.1152/ajpheart.00221.2012. Epub 2012 May 25. Am J Physiol Heart Circ Physiol. 2012. PMID: 22636682 Free PMC article. Review.
-
Are nestin-positive mesenchymal stromal cells a better source of cells for CNS repair?Neurochem Int. 2017 Jun;106:101-107. doi: 10.1016/j.neuint.2016.08.001. Epub 2016 Aug 3. Neurochem Int. 2017. PMID: 27498150 Free PMC article. Review.
-
Isolation and characterization of multipotent mesenchymal stem cells in nasal polyps.Exp Biol Med (Maywood). 2015 Feb;240(2):185-93. doi: 10.1177/1535370214553898. Epub 2014 Oct 6. Exp Biol Med (Maywood). 2015. PMID: 25294891 Free PMC article. Clinical Trial.
References
-
- Dazzi F. Ramasamy R. Glennie S. Jones SP. Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. - PubMed
-
- Palsson BO. Paek SH. Schwartz RM. Palsson M. Lee GM. Silver S. Emerson SG. Expansion of human bone marrow progenitor cells in a high cell density continuous perfusion system. Bio/Technology. 1993;11:368–372. - PubMed
-
- Cabrita GJM. Ferreira BS. Silva CL. Goncalves R. Almeida-Porada G. Cabral JMS. Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol. 2003;21:233–240. - PubMed
-
- Bernstein ID. Andrews RG. Zsebo KM. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+ lin- cells, and the generation of colony-formation cell progeny from CD34+ lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:2316–2321. - PubMed
-
- Lowry PA. Zsebo KM. Deacon DH. Eichman CE. Quesenberry PJ. Effects of rhSCF on multiple cytokine-responsive HPP-CFC generated from Sca-1+ Lin- murine hematopoietic progenitors. Exp Hematol. 1991;19:994–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

