Production and validation of a good manufacturing practice grade human fibroblast line for supporting human embryonic stem cell derivation and culture
- PMID: 22472092
- PMCID: PMC3392772
- DOI: 10.1186/scrt103
Production and validation of a good manufacturing practice grade human fibroblast line for supporting human embryonic stem cell derivation and culture
Abstract
Introduction: The development of reproducible methods for deriving human embryonic stem cell (hESC) lines in compliance with good manufacturing practice (GMP) is essential for the development of hESC-based therapies. Although significant progress has been made toward the development of chemically defined conditions for the maintenance and differentiation of hESCs, efficient derivation of new hESCs requires the use of fibroblast feeder cells. However, GMP-grade feeder cell lines validated for hESC derivation are not readily available.
Methods: We derived a fibroblast cell line (NclFed1A) from human foreskin in compliance with GMP standards. Consent was obtained to use the cells for the production of hESCs and to generate induced pluripotent stem cells (iPSCs). We compared the line with a variety of other cell lines for its ability to support derivation and self-renewal of hESCs.
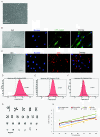
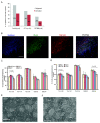
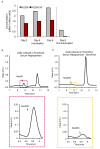
Results: NclFed1A supports efficient rates (33%) of hESC colony formation after explantation of the inner cell mass (ICM) of human blastocysts. This compared favorably with two mouse embryonic fibroblast (MEF) cell lines. NclFed1A also compared favorably with commercially available foreskin fibroblasts and MEFs in promoting proliferation and pluripotency of a number of existing and widely used hESCs. The ability of NclFed1A to maintain self-renewal remained undiminished for up to 28 population doublings from the master cell bank.
Conclusions: The human fibroblast line Ncl1Fed1A, produced in compliance with GMP standards and qualified for derivation and maintenance of hESCs, is a useful resource for the advancement of progress toward hESC-based therapies in regenerative medicine.
Figures
Similar articles
-
Generation of Sheffield (Shef) human embryonic stem cell lines using a microdrop culture system.In Vitro Cell Dev Biol Anim. 2010 Apr;46(3-4):236-41. doi: 10.1007/s11626-010-9294-2. Epub 2010 Mar 12. In Vitro Cell Dev Biol Anim. 2010. PMID: 20224972
-
High quality clinical grade human embryonic stem cell lines derived from fresh discarded embryos.Stem Cell Res Ther. 2017 Jun 5;8(1):128. doi: 10.1186/s13287-017-0561-y. Stem Cell Res Ther. 2017. PMID: 28583200 Free PMC article.
-
Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture.Stem Cell Res. 2012 Jan;8(1):97-108. doi: 10.1016/j.scr.2011.08.005. Epub 2011 Aug 27. Stem Cell Res. 2012. PMID: 22099024
-
Derivation of Human Skin Fibroblast Lines for Feeder Cells of Human Embryonic Stem Cells.Curr Protoc Stem Cell Biol. 2016 Feb 3;36:1C.7.1-1C.7.11. doi: 10.1002/9780470151808.sc01c07s36. Curr Protoc Stem Cell Biol. 2016. PMID: 26840224 Review.
-
Embryonic stem cells: isolation, characterization and culture.Adv Biochem Eng Biotechnol. 2009;114:173-84. doi: 10.1007/10_2008_20. Adv Biochem Eng Biotechnol. 2009. PMID: 19495683 Review.
Cited by
-
Feeder & basic fibroblast growth factor-free culture of human embryonic stem cells: Role of conditioned medium from immortalized human feeders.Indian J Med Res. 2016 Dec;144(6):838-851. doi: 10.4103/ijmr.IJMR_424_15. Indian J Med Res. 2016. PMID: 28474621 Free PMC article.
-
Two decades of embryonic stem cells: a historical overview.Hum Reprod Open. 2019 Jan 29;2019(1):hoy024. doi: 10.1093/hropen/hoy024. eCollection 2019. Hum Reprod Open. 2019. PMID: 30895264 Free PMC article.
-
1H NMR Metabolite Monitoring during the Differentiation of Human Induced Pluripotent Stem Cells Provides New Insights into the Molecular Events That Regulate Embryonic Chondrogenesis.Int J Mol Sci. 2022 Aug 17;23(16):9266. doi: 10.3390/ijms23169266. Int J Mol Sci. 2022. PMID: 36012540 Free PMC article.
-
Defining synthetic surfaces for human pluripotent stem cell culture.Cell Regen. 2013 Nov 22;2(1):7. doi: 10.1186/2045-9769-2-7. eCollection 2013. Cell Regen. 2013. PMID: 25408879 Free PMC article. Review.
-
The Molecular Karyotype of 25 Clinical-Grade Human Embryonic Stem Cell Lines.Sci Rep. 2015 Nov 26;5:17258. doi: 10.1038/srep17258. Sci Rep. 2015. PMID: 26607962 Free PMC article.
References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Catalina P, Montes R, Ligero G, Sanchez L, de la Cueva T, Bueno C, Leone PE, Menendez P. Human ESCs predisposition to karyotypic instability: is it a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer. 2008;7:76. doi: 10.1186/1476-4598-7-76. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

