Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles
- PMID: 22472443
- PMCID: PMC3317806
- DOI: 10.1083/jcb.201111049
Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles
Abstract
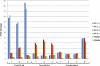
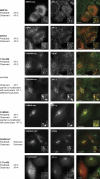
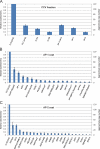
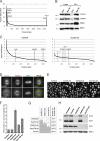
Despite recent advances in mass spectrometry, proteomic characterization of transport vesicles remains challenging. Here, we describe a multivariate proteomics approach to analyzing clathrin-coated vesicles (CCVs) from HeLa cells. siRNA knockdown of coat components and different fractionation protocols were used to obtain modified coated vesicle-enriched fractions, which were compared by stable isotope labeling of amino acids in cell culture (SILAC)-based quantitative mass spectrometry. 10 datasets were combined through principal component analysis into a "profiling" cluster analysis. Overall, 136 CCV-associated proteins were predicted, including 36 new proteins. The method identified >93% of established CCV coat proteins and assigned >91% correctly to intracellular or endocytic CCVs. Furthermore, the profiling analysis extends to less well characterized types of coated vesicles, and we identify and characterize the first AP-4 accessory protein, which we have named tepsin. Finally, our data explain how sequestration of TACC3 in cytosolic clathrin cages causes the severe mitotic defects observed in auxilin-depleted cells. The profiling approach can be adapted to address related cell and systems biological questions.
Figures




References
-
- Abou Jamra R., Philippe O., Raas-Rothschild A., Eck S.H., Graf E., Buchert R., Borck G., Ekici A., Brockschmidt F.F., Nöthen M.M., et al. 2011. Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 88:788–795 10.1016/j.ajhg.2011.04.019 - DOI - PMC - PubMed
-
- Blondeau F., Ritter B., Allaire P.D., Wasiak S., Girard M., Hussain N.K., Angers A., Legendre-Guillemin V., Roy L., Boismenu D., et al. 2004. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA. 101:3833–3838 10.1073/pnas.0308186101 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

