Male risk taking, female odors, and the role of estrogen receptors
- PMID: 22472459
- PMCID: PMC4777405
- DOI: 10.1016/j.physbeh.2012.03.017
Male risk taking, female odors, and the role of estrogen receptors
Abstract
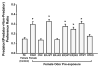
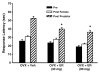
Male risk-taking and decision making are affected by sex-related cues, with men making riskier choices and decisions after exposure to either women or stimuli associated with women. In non-human species females and, or their cues can also increase male risk taking. Under the ecologically relevant condition of predation threat, brief exposure of male mice to the odors of a sexually receptive novel female reduces the avoidance of, and aversive responses to, a predator. We briefly review evidence showing that estrogen receptors (ERs), ERα and ERβ, are associated with the mediation of these risk taking responses. We show that ERs influence the production of the female odors that affect male risk taking, with the odors of wild type (ERαWT, ERβWT), oxytocin (OT) wildtype (OTWT), gene-deleted 'knock-out' ERβ (ERβKO), but not ERαKO or oxytocin (OT) OTKO or ovariectomized (OVX) female mice reducing the avoidance responses of male mice to cat odor. We further show that administration of specific ERα and ERβ agonists to OVX females results in their odors increasing male risk taking and boldness towards a predator. We also review evidence that ERs are involved in the mediation of the responses of males to female cues, with ERα being associated with the sexual and both ERβ and ERα with the sexual and social mechanisms underlying the effects of female cues on male risk taking. The implications and relations of these findings with rodents to ERs and the regulation of human risk taking are briefly considered.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Roney JR. Effects of visual exposure to the opposite sex: cognitive aspects of mate attraction in human males. Pers Soc Psychol Bull. 2006;29:393–404. - PubMed
-
- Roney JR, Mahler SV, Maestripieri D. Behavioral and hormonal responses of men to brief interactions with women. Evol Human Behav. 2003;21:1–9.
-
- Ariely D, Lowenstein G. The heat of the moment: the effect of sexual arousal on sexual decision making. J Behav Decis Making. 2006;19:87–98.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

