Deletion of astroglial connexins weakens the blood-brain barrier
- PMID: 22472609
- PMCID: PMC3421093
- DOI: 10.1038/jcbfm.2012.45
Deletion of astroglial connexins weakens the blood-brain barrier
Abstract
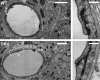
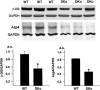
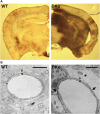
Astrocytes, the most prominent glial cell type in the brain, send specialized processes named endfeet, which enwrap blood vessels and express a large molecular repertoire dedicated to the physiology of the vascular system. One of the most striking properties of astrocyte endfeet is their enrichment in gap junction protein connexins 43 and 30 (Cx43 and Cx30) allowing for direct intercellular trafficking of ions and small signaling molecules through perivascular astroglial networks. The contribution of astroglial connexins to the physiology of the brain vascular system has never been addressed. Here, we show that Cx43 and Cx30 expression at the level of perivascular endfeet starts from postnatal days 2 and 12 and is fully mature at postnatal days 15 and 20, respectively, indicating that astroglial perivascular connectivity occurs and develops during postnatal blood-brain barrier (BBB) maturation. We demonstrate that mice lacking Cx30 and Cx43 in GFAP (glial fibrillary acidic protein)-positive cells display astrocyte endfeet edema and a partial loss of the astroglial water channel aquaporin-4 and β-dystroglycan, a transmembrane receptor anchoring astrocyte endfeet to the perivascular basal lamina. Furthermore, the absence of astroglial connexins weakens the BBB, which opens upon increased hydrostatic vascular pressure and shear stress. These results demonstrate that astroglial connexins are necessary to maintain BBB integrity.
Figures



Comment in
-
Making connexons in the neurovascular unit.J Cereb Blood Flow Metab. 2012 Aug;32(8):1455-6. doi: 10.1038/jcbfm.2012.44. Epub 2012 Apr 4. J Cereb Blood Flow Metab. 2012. PMID: 22472614 Free PMC article. No abstract available.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Dagenais C, Rousselle C, Pollack GM, Scherrmann JM. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J Cereb Blood Flow Metab. 2000;20:381–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

