High-resolution structure of infectious prion protein: the final frontier
- PMID: 22472622
- PMCID: PMC4049221
- DOI: 10.1038/nsmb.2266
High-resolution structure of infectious prion protein: the final frontier
Abstract
Prions are the proteinaceous infectious agents responsible for the transmission of prion diseases. The main or sole component of prions is the misfolded prion protein (PrP(Sc)), which is able to template the conversion of the host's natively folded form of the protein (PrP(C)). The detailed mechanism of prion replication and the high-resolution structure of PrP(Sc) are unknown. The currently available information on PrP(Sc) structure comes mostly from low-resolution biophysical techniques, which have resulted in quite divergent models. Recent advances in the production of infectious prions, using very pure recombinant protein, offer new hope for PrP(Sc) structural studies. This review highlights the importance of, challenges for and recent progress toward elucidating the elusive structure of PrP(Sc), arguably the major pending milestone to reach in understanding prions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
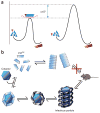
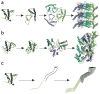
Similar articles
-
Molecular Mechanism of the Misfolding and Oligomerization of the Prion Protein: Current Understanding and Its Implications.Biochemistry. 2015 Jul 28;54(29):4431-42. doi: 10.1021/acs.biochem.5b00605. Epub 2015 Jul 17. Biochemistry. 2015. PMID: 26171558 Review.
-
Quaternary structure of pathological prion protein as a determining factor of strain-specific prion replication dynamics.PLoS Pathog. 2013;9(10):e1003702. doi: 10.1371/journal.ppat.1003702. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130496 Free PMC article.
-
Toward a mechanism of prion misfolding and structural models of PrP(Sc): current knowledge and future directions.J Toxicol Environ Health A. 2011;74(2-4):154-60. doi: 10.1080/15287394.2011.529065. J Toxicol Environ Health A. 2011. PMID: 21218344 Review.
-
New insights into prion structure and toxicity.Neuron. 2006 May 4;50(3):353-7. doi: 10.1016/j.neuron.2006.04.020. Neuron. 2006. PMID: 16675391 Review.
-
Protein misfolding cyclic amplification of prions.J Vis Exp. 2012 Nov 7;(69):4075. doi: 10.3791/4075. J Vis Exp. 2012. PMID: 23168797 Free PMC article.
Cited by
-
Attachment of pathogenic prion protein to model oxide surfaces.Environ Sci Technol. 2013 Jul 2;47(13):6925-34. doi: 10.1021/es3045899. Epub 2013 May 30. Environ Sci Technol. 2013. PMID: 23611152 Free PMC article.
-
Highly infectious prions generated by a single round of microplate-based protein misfolding cyclic amplification.mBio. 2013 Dec 31;5(1):e00829-13. doi: 10.1128/mBio.00829-13. mBio. 2013. PMID: 24381300 Free PMC article.
-
Genetic prion disease-related mutation E196K displays a novel amyloid fibril structure revealed by cryo-EM.Sci Adv. 2021 Sep 10;7(37):eabg9676. doi: 10.1126/sciadv.abg9676. Epub 2021 Sep 8. Sci Adv. 2021. PMID: 34516876 Free PMC article.
-
New and distinct chronic wasting disease strains associated with cervid polymorphism at codon 116 of the Prnp gene.PLoS Pathog. 2021 Jul 26;17(7):e1009795. doi: 10.1371/journal.ppat.1009795. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34310662 Free PMC article.
-
Mammalian prion amyloid formation in bacteria.Prion. 2016 Mar 3;10(2):112-8. doi: 10.1080/19336896.2016.1141859. Prion. 2016. PMID: 26910379 Free PMC article.
References
-
- Delez AL, Gustafson DP, Luttrell CN. Some clinical and histological observations on scrapie in sheep. J Am Vet Med Assoc. 1957;131:439–446. - PubMed
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Alper T. Scrapie agent unlike viruses in size and susceptibility to inactivation by ionizing or ultraviolet radiation. Nature. 1985;317:750. - PubMed
-
- Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. - PubMed
-
- Hope J, Ritchie L, Farquhar C, Somerville R, Hunter N. Bovine spongiform encephalopathy: a scrapie-like disease of British cattle. Prog Clin Biol Res. 1989;317:659–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

