A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing
- PMID: 22472650
- PMCID: PMC3699424
- DOI: 10.1126/scisignal.2002549
A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing
Abstract
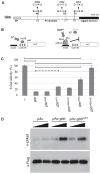
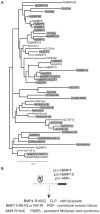
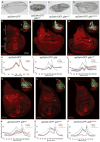
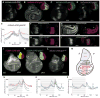
Dimers of conventional transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) ligands are composed of two 100- to 140-amino acid peptides that are produced through the proteolytic processing of a proprotein precursor by proconvertases, such as furin. We report the identification of an evolutionarily conserved furin processing site in the amino terminus (NS) of the Glass bottom boat (Gbb; the Drosophila ortholog of vertebrate BMP5, 6, and 7) proprotein that generates a 328-amino acid, active BMP ligand distinct from the conventional 130-amino acid ligand. Gbb38, the large ligand form of Gbb, exhibited greater signaling activity and a longer range than the shorter form Gbb15. The abundance of Gbb15 and Gbb38 varied among different tissues, raising the possibility that differential processing could account for tissue-specific behaviors of BMPs. In human populations, mutations that abolished the NS cleavage site in BMP4, BMP15, or anti-Müllerian hormone were associated with cleft lip with or without cleft palate (BMP4), premature ovarian failure (BMP15), and persistent Müllerian duct syndrome (anti-Müllerian hormone), suggesting the importance of NS processing during development. The identification of this large BMP ligand form and the functional differences between large and small ligands exemplifies the potential for differential proprotein processing to substantially affect BMP and TGF-β signaling output in different tissue and cellular contexts.
Conflict of interest statement
Figures


References
-
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. - PubMed
-
- Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
-
- Wu MY, Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. - PubMed
-
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: From pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. - PubMed
-
- Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006;295:178–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

