IRAK-M modulates expression of IL-10 and cell surface markers CD80 and MHC II after bacterial re-stimulation of tolerized dendritic cells
- PMID: 22472665
- PMCID: PMC3577058
- DOI: 10.1016/j.imlet.2012.03.006
IRAK-M modulates expression of IL-10 and cell surface markers CD80 and MHC II after bacterial re-stimulation of tolerized dendritic cells
Abstract
Background: As essential components of the innate immune system, dendritic cells (DCs) can interact directly with pathogens as well as participate in the adaptive immune response. In cells closely related to DCs such as macrophages and monocytes, prior exposure to minute amounts of endotoxin can lead to a refractory period where subsequent exposure to higher doses fails to induce an inflammatory response; little research has investigated this effect on DCs. This study tested if murine bone marrow-derived dendritic cells (BM-DCs) respond to endotoxin- and bacterial sonicate-induced tolerance by decreased inflammatory and increased anti-inflammatory response, and the role of IRAK-M, an intracellular negative regulator of TLR signaling, in this tolerance.
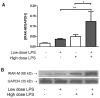
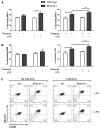
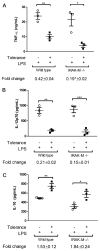
Results: Tolerized BM-DCs exhibited a significant drop in TNF-α and IL-12p70 production and increased IL-10 expression compared to untolerized cells. BM-DCs also showed the ability to develop heterotolerance, in which the LPS exposure alone was able to induce tolerance to Helicobacter pylori sonicate and TLR2 agonist Pam3Cys. Furthermore, the expression of IRAK-M was increased after restimulation of tolerized BM-DCs as determined qPCR and Western blot. IRAK-M exhibited a suppressive effect on surface expression of major histocompatibilty complex class II (MHC II) and CD80 in LPS-tolerized BM-DCs. IL-10 expression in bacterial sonicate-tolerized IRAK-M-/- BM-DCs was altered as compared to wild type BM-DCs, with tolerance-induced expression of IL-10 mitigated in tolerized IRAK-M-/- BM-DCs.
Conclusion: Along with endotoxin, bacterial sonicate is able to induce refractory tolerance in BM-DCs, and IRAK-M plays a role in modulating cell surface expression of MHC class II and CD80 and release of IL-10 during this tolerance.
Copyright © 2012 Elsevier B.V. All rights reserved.
Conflict of interest statement
There is no conflict of interest declared for any author.
Figures




References
-
- Cherayil BJ. How not to get bugged by bugs: mechanisms of cellular tolerance to microorganisms. Curr Opin Gastroenterol. 2003;19:572–7. - PubMed
-
- Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. - PubMed
-
- Liu ZJ, Yan LN, Li XH, Xu FL, Chen XF, You HB, et al. Up-regulation of IRAK-M is essential for endotoxin tolerance induced by a low dose of lipopolysaccharide in Kupffer cells. J Surg Res. 2008;150:34–9. - PubMed
-
- del Fresno C, Garcia-Rio F, Gomez-Pina V, Soares-Schanoski A, Fernandez-Ruiz I, Jurado T, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol. 2009;182:6494–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

