Dynamic equilibrium between cancer stem cells and non-stem cancer cells in human SW620 and MCF-7 cancer cell populations
- PMID: 22472879
- PMCID: PMC3341854
- DOI: 10.1038/bjc.2012.126
Dynamic equilibrium between cancer stem cells and non-stem cancer cells in human SW620 and MCF-7 cancer cell populations
Abstract
Background: Cancer stem cells (CSCs) paradigm suggests that CSCs might have important clinical implications in cancer therapy. Previously, we reported that accumulation efficiency of CSCs is different post low- and high-LET irradiation in 48 h.
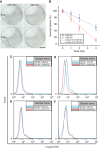
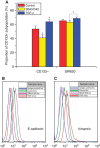
Methods: Cancer stem cells and non-stem cancer cells (NSCCs) were sorted and functionally identified through a variety of assays such as antigen profiles and sphere formation. Inter-conversion between CSCs and NSCCs were in situ visualised. Cancer stem cells proportions were assayed over multiple generations under normal and irradiation surroundings. Supplement and inhibition of TGF-β1, as well as immunofluorescence assay of E-cadherin and Vimentin, were performed.
Results: Surface antigen markers of CSCs and NSCCs exist in an intrinsic homoeostasis state with spontaneous and in situ visualisable inter-conversions, irrespective of prior radiations. Supplement with TGF-β1 accelerates the equilibrium, whereas inhibition of TGF-β signalling disturbs the equilibrium and significantly decreases CSC proportion. Epithelial mesenchymal transition (EMT) might be activated during the process.
Conclusion: Our results indicate that the intrinsic inter-conversion and dynamic equilibrium between CSCs and NSCCs exist under normal and irradiation surroundings, and TGF-β might have important roles in the equilibrium through activating EMT.
Figures



References
-
- Adams JM, Kelly PN, Dakic A, Nutt SL, Strasser A (2007) Response to comment on ‘tumor growth need not be driven by rare cancer stem cells’. Science 318(5857): 1722 - PubMed
-
- Alison MR, Lim SML, Nicholson LJ (2011) Cancer stem cells: problems for therapy? J Pathol 223(2): 148–162 - PubMed
-
- Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, Bissell MJ, Barcellos-Hoff MH (2007) Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res 67(18): 8662–8670 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

