Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers
- PMID: 22472995
- PMCID: PMC3837382
- DOI: 10.1038/clpt.2011.323
Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers
Abstract
Rifapentine (RP T) is an antituberculosis drug that may shorten treatment duration when substituted for rifampin (RI F).The maximal tolerated daily dose of RP T and its potential for cytochrome 3A4 induction and autoinduction at clinically relevant doses are unknown. In this phase I, dose-escalation study among healthy volunteers, daily doses as high asa prespecified maximum of 20 mg/kg/day were well tolerated. Steady-state RP T concentrations increased with dose from 5 to 15 mg/kg, but area under the plasma concentration–time curve (AU C0–24) and maximum concentration (Cmax)were similar in the 15- and 20-mg/kg cohorts. Although RP T pharmacokinetics (PK) appeared to be time-dependent,accumulation occurred with daily dosing. The mean AU C0–12 of oral midazolam (MDZ), a cytochrome 3A (CYP 3A) probe drug, was reduced by 93% with the coadministration of RPT and by 74% with the coadministration of RIF (P < 0.01).Changes in the oral clearance of MDZ did not vary by RP T dose. In conclusion, RP T was tolerated at doses as high as20 mg/kg/day, its PK were less than dose-proportional, and its CYP 3A induction was robust.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

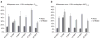

Similar articles
-
Evaluation of the pharmacokinetic interaction between repeated doses of rifapentine or rifampin and a single dose of bedaquiline in healthy adult subjects.Antimicrob Agents Chemother. 2015 Feb;59(2):1219-24. doi: 10.1128/AAC.04171-14. Epub 2014 Dec 15. Antimicrob Agents Chemother. 2015. PMID: 25512422 Free PMC article. Clinical Trial.
-
Enzyme induction observed in healthy volunteers after repeated administration of rifapentine and its lack of effect on steady-state rifapentine pharmacokinetics: part I.Int J Tuberc Lung Dis. 1999 May;3(5):426-36. Int J Tuberc Lung Dis. 1999. PMID: 10331733 Clinical Trial.
-
Novel dosing strategies increase exposures of the potent antituberculosis drug rifapentine but are poorly tolerated in healthy volunteers.Antimicrob Agents Chemother. 2015;59(6):3399-405. doi: 10.1128/AAC.05128-14. Epub 2015 Mar 30. Antimicrob Agents Chemother. 2015. PMID: 25824215 Free PMC article. Clinical Trial.
-
Rifapentine: its role in the treatment of tuberculosis.Ann Pharmacother. 1999 Nov;33(11):1203-10. doi: 10.1345/aph.18450. Ann Pharmacother. 1999. PMID: 10573321 Review.
-
Lurasidone drug-drug interaction studies: a comprehensive review.Drug Metabol Drug Interact. 2014;29(3):191-202. doi: 10.1515/dmdi-2014-0005. Drug Metabol Drug Interact. 2014. PMID: 24825095 Review.
Cited by
-
Use of integrase inhibitors in HIV-associated tuberculosis in high-burden settings: implementation challenges and research gaps.Lancet HIV. 2022 Feb;9(2):e130-e138. doi: 10.1016/S2352-3018(21)00324-6. Lancet HIV. 2022. PMID: 35120633 Free PMC article. Review.
-
Alternatives to rifampicin: A review and perspectives on the choice of strong CYP3A inducers for clinical drug-drug interaction studies.Clin Transl Sci. 2022 Sep;15(9):2075-2095. doi: 10.1111/cts.13357. Epub 2022 Jul 25. Clin Transl Sci. 2022. PMID: 35722783 Free PMC article. Review.
-
Therapeutic drug monitoring in tuberculosis.Eur J Clin Pharmacol. 2024 Nov;80(11):1659-1684. doi: 10.1007/s00228-024-03749-8. Epub 2024 Sep 6. Eur J Clin Pharmacol. 2024. PMID: 39240337 Review.
-
Evaluation of the pharmacokinetic interaction between repeated doses of rifapentine or rifampin and a single dose of bedaquiline in healthy adult subjects.Antimicrob Agents Chemother. 2015 Feb;59(2):1219-24. doi: 10.1128/AAC.04171-14. Epub 2014 Dec 15. Antimicrob Agents Chemother. 2015. PMID: 25512422 Free PMC article. Clinical Trial.
-
Drug Release Characteristics and Tissue Distribution of Rifapentine Polylactic Acid Sustained-Release Microspheres in Rabbits after Paravertebral Implantation.Iran Red Crescent Med J. 2016 Aug 13;18(11):e38661. doi: 10.5812/ircmj.38661. eCollection 2016 Nov. Iran Red Crescent Med J. 2016. PMID: 28210500 Free PMC article.
References
-
- World Health Organization. Global Tuberculosis Control: WHO report 2010. WHO Press; Geneva, Switzerland: 2010.
-
- Bemer-Melchior P, Bryskier A, Drugeon HB. Comparison of the in vitro activities of rifapentine and rifampicin against Mycobacterium tuberculosis complex. J Antimicrob Chemother. 2000;46:571–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

