5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy
- PMID: 22473220
- PMCID: PMC3487141
- DOI: 10.1159/000337918
5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy
Abstract
Background/aims: We previously reported that patients with chronic kidney disease (CKD) receiving warfarin therapy and whose international normalized ratio increases to >3.0 may develop acute kidney injury (AKI) as a result of glomerular hemorrhage and formation of obstructive red blood cell (RBC) casts. We named this condition warfarin-related nephropathy (WRN). We also previously reported that acute excessive anticoagulation with brodifacoum (superwarfarin) induces AKI in 5/6 nephrectomy (5/6NE) rats. Limitations of the brodifacoum model precluded a careful assessment of dose-response relationships.
Methods: Warfarin treatment was used in 5/6NE.
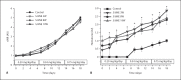
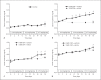
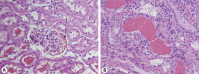
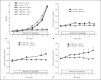
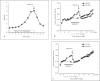
Results: Herein we report that warfarin treatment of 5/6NE rats resulted in a dose-dependent increase in serum creatinine (SC). The increase in SC following warfarin treatment was greater at 3 and 19 weeks after the ablative surgery, than that observed 8 weeks after the ablative surgery. The SC increase was correlated with the prothrombin time increase. Morphologically, 5/6NE, but not control rats, had acute tubular injury with RBC and RBC casts in the tubules. Treatment with vitamin K prevented SC increase and morphologic changes in the kidney associated with warfarin treatment. A single episode of WRN did not affect the progression of CKD in 5/6NE.
Conclusion: (1) The 5/6NE model of CKD is an appropriate animal model to study the pathogenesis of WRN. (2) The pharmacokinetics of warfarin is better suited to the study of WRN than that of brodifacoum. (3) The more advanced stages of 5/6NE are more susceptible to WRN than the earlier stages. (4) Vitamin K treatment prevents WRN.
Copyright © 2012 S. Karger AG, Basel.
Figures
Similar articles
-
Research Letter: Is the 129S1/SvImJ Mouse Strain More Suitable to Study Anticoagulant-Related Nephropathy Than the C57BL/6 Strain?Can J Kidney Health Dis. 2023 Mar 16;10:20543581231160507. doi: 10.1177/20543581231160507. eCollection 2023. Can J Kidney Health Dis. 2023. PMID: 36950027 Free PMC article.
-
N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy.Am J Physiol Renal Physiol. 2013 Jun 15;304(12):F1421-7. doi: 10.1152/ajprenal.00689.2012. Epub 2013 Apr 10. Am J Physiol Renal Physiol. 2013. PMID: 23576637
-
Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats.Nephrol Dial Transplant. 2014 Dec;29(12):2228-34. doi: 10.1093/ndt/gft380. Epub 2013 Sep 5. Nephrol Dial Transplant. 2014. PMID: 24009280
-
Anticoagulants and acute kidney injury: clinical and pathology considerations.Kidney Res Clin Pract. 2014 Dec;33(4):174-80. doi: 10.1016/j.krcp.2014.11.001. Epub 2014 Nov 18. Kidney Res Clin Pract. 2014. PMID: 26885473 Free PMC article. Review.
-
The use of vitamin K in patients on anticoagulant therapy: a practical guide.Am J Cardiovasc Drugs. 2004;4(1):43-55. doi: 10.2165/00129784-200404010-00005. Am J Cardiovasc Drugs. 2004. PMID: 14967065 Review.
Cited by
-
Supratherapeutic International Normalized Ratio causing Nephropathy: A Rare Adverse Effect of Warfarin.Cureus. 2019 Jul 22;11(7):e5201. doi: 10.7759/cureus.5201. Cureus. 2019. PMID: 31565607 Free PMC article.
-
Kidney disease models: tools to identify mechanisms and potential therapeutic targets.Zool Res. 2018 Mar 18;39(2):72-86. doi: 10.24272/j.issn.2095-8137.2017.055. Zool Res. 2018. PMID: 29515089 Free PMC article. Review.
-
The Impairment in Kidney Function in the Oral Anticoagulation Era. A Pathophysiological Insight.Cardiovasc Drugs Ther. 2021 Jun;35(3):505-519. doi: 10.1007/s10557-020-07004-x. Cardiovasc Drugs Ther. 2021. PMID: 32535717
-
Research Letter: Is the 129S1/SvImJ Mouse Strain More Suitable to Study Anticoagulant-Related Nephropathy Than the C57BL/6 Strain?Can J Kidney Health Dis. 2023 Mar 16;10:20543581231160507. doi: 10.1177/20543581231160507. eCollection 2023. Can J Kidney Health Dis. 2023. PMID: 36950027 Free PMC article.
-
Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling.Int J Clin Exp Pathol. 2015 May 1;8(5):4725-34. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191162 Free PMC article.
References
-
- Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. - PubMed
-
- Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–c146. - PMC - PubMed
-
- Subject Revision of the Guide for the Care and Use of Laboratory Animals . Bethesda, Md. U.S. Department of Health and Human Services Public Health Services: National Institutes of Health, Publication Number; 1985. pp. 86–23.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

