Association of a protective monoclonal IgA with the O antigen of Salmonella enterica serovar Typhimurium impacts type 3 secretion and outer membrane integrity
- PMID: 22473607
- PMCID: PMC3416483
- DOI: 10.1128/IAI.00018-12
Association of a protective monoclonal IgA with the O antigen of Salmonella enterica serovar Typhimurium impacts type 3 secretion and outer membrane integrity
Abstract
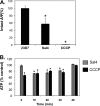
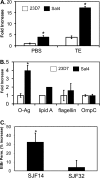
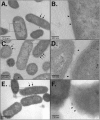
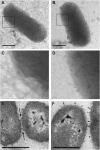
Invasion of intestinal epithelial cells by Salmonella enterica serovar Typhimurium is an energetically demanding process, involving the transfer of effector proteins from invading bacteria into host cells via a specialized organelle known as the Salmonella pathogenicity island 1 (SPI-1) type 3 secretion system (T3SS). By a mechanism that remains poorly understood, entry of S. Typhimurium into epithelial cells is inhibited by Sal4, a monoclonal, polymeric IgA antibody that binds an immunodominant epitope within the O-antigen (O-Ag) component of lipopolysaccharide. In this study, we investigated how the binding of Sal4 to the surface of S. Typhimurium influences T3SS activity, bacterial energetics, and outer membrane integrity. We found that Sal4 treatment impaired T3SS-mediated translocon formation and attenuated the delivery of tagged effector proteins into epithelial cells. Sal4 treatment coincided with a partial reduction in membrane energetics and intracellular ATP levels, possibly explaining the impairment in T3SS activity. Sal4's effects on bacterial secretion and energetics occurred concurrently with an increase in O-Ag levels in culture supernatants, alterations in outer membrane permeability, and changes in surface ultrastructure, as revealed by transmission electron microscopy and cryo-electron microscopy. We propose that Sal4, by virtue of its ability to bind and cross-link the O-Ag, induces a form of outer membrane stress that compromises the integrity of the S. Typhimurium cell envelope and temporarily renders the bacterium avirulent.
Figures


References
-
- Bakowski MA, et al. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe 7:453–462 - PubMed
-
- Becker LA, Bang IS, Crouch ML, Fang FC. 2005. Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol. Microbiol. 56:1004–1016 - PubMed
-
- Berg HC. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

