Naphthoquinone components from Alkanna tinctoria (L.) Tausch show significant antiproliferative effects on human colorectal cancer cells
- PMID: 22473633
- PMCID: PMC3475751
- DOI: 10.1002/ptr.4680
Naphthoquinone components from Alkanna tinctoria (L.) Tausch show significant antiproliferative effects on human colorectal cancer cells
Abstract
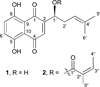
Our research to seek active compounds against human colorectal cancer from the root of Alkanna tinctoria (L.) Tausch led to the isolation of two naphthoquinones, alkannin (1) and angelylalkannin (2). The antiproliferative effects of the two compounds on human colon cancer cells HCT-116 and SW-480 were determined by the 3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) method. Cell cycle profile and cell apoptosis were determined using flow cytometry. Both of the two compounds showed significant inhibitory effects on the cancer cells. For alkannin (1) and angelylalkannin (2), the median inhibitory concentration (IC₅₀) values were 2.38 and 4.76 µM for HCT-116 cells, while for SW-480 cells they were 4.53 and 7.03 µM, respectively. The potential antiproliferative mechanisms were also explored. At concentrations between 1-10 µM, both compounds arrested the cell cycle at the G1 phase and induced cell apoptosis.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures



Similar articles
-
Isolation and chemopreventive evaluation of novel naphthoquinone compounds from Alkanna tinctoria.Anticancer Drugs. 2013 Nov;24(10):1058-68. doi: 10.1097/CAD.0000000000000017. Anticancer Drugs. 2013. PMID: 24025561 Free PMC article.
-
Naphthoquinone rich Onosma visianii Clem (Boraginaceae) root extracts induce apoptosis and cell cycle arrest in HCT-116 and MDA-MB-231 cancer cell lines.Nat Prod Res. 2018 Nov;32(22):2712-2716. doi: 10.1080/14786419.2017.1374271. Epub 2017 Sep 7. Nat Prod Res. 2018. PMID: 28882053
-
Radical scavenging activity of Alkanna tinctoria root extracts and their main constituents, hydroxynaphthoquinones.Phytother Res. 2005 Feb;19(2):141-7. doi: 10.1002/ptr.1645. Phytother Res. 2005. PMID: 15852495
-
Investigation of the synergistic effects of paclitaxel and herbal substances and endemic plant extracts on cell cycle and apoptosis signal pathways in prostate cancer cell lines.Gene. 2019 Mar 1;687:261-271. doi: 10.1016/j.gene.2018.11.049. Epub 2018 Nov 17. Gene. 2019. PMID: 30453074
-
Cytotoxic naphthoquinones from Alkanna cappadocica ( perpendicular).J Nat Prod. 2010 May 28;73(5):860-4. doi: 10.1021/np900778j. J Nat Prod. 2010. PMID: 20405844
Cited by
-
Pharmacological and analytical aspects of alkannin/shikonin and their derivatives: An update from 2008 to 2022.Chin Herb Med. 2022 Sep 20;14(4):511-527. doi: 10.1016/j.chmed.2022.08.001. eCollection 2022 Oct. Chin Herb Med. 2022. PMID: 36405061 Free PMC article. Review.
-
Synthesis and cytotoxic evaluation of a series of 2-amino-naphthoquinones against human cancer cells.Molecules. 2014 Aug 26;19(9):13188-99. doi: 10.3390/molecules190913188. Molecules. 2014. PMID: 25162959 Free PMC article.
-
Anti-Proliferative Activities and Apoptosis Induction by Triterpenes Derived from Eriobotrya japonica in Human Leukemia Cell Lines.Int J Mol Sci. 2013 Feb 18;14(2):4106-20. doi: 10.3390/ijms14024106. Int J Mol Sci. 2013. PMID: 23429195 Free PMC article.
-
Quassinoids from the Root of Eurycoma longifolia and Their Antiproliferative Activity on Human Cancer Cell Lines.Pharmacogn Mag. 2017 Jul-Sep;13(51):459-462. doi: 10.4103/pm.pm_353_16. Epub 2017 Jul 19. Pharmacogn Mag. 2017. PMID: 28839372 Free PMC article.
-
Metabolic Profiles, Genetic Diversity, and Genome Size of Bulgarian Population of Alkanna tinctoria.Plants (Basel). 2022 Dec 26;12(1):111. doi: 10.3390/plants12010111. Plants (Basel). 2022. PMID: 36616241 Free PMC article.
References
-
- Akerele O. Nature's medicinal bounty: don't throw it away. World Health Forum. 1993;14:390–395. - PubMed
-
- Ali A, Assimopoulou AN, Papageorgiou VP, Kolodziej H. Structure/antileishmanial activity relationship study of naphthoquinones and dependency of the mode of action on the substitution patterns. Planta Med. 2011;77:2003–2012. - PubMed
-
- Assimopoulou AN, Papageorgiou VP. Radical scavenging activity of Alkanna tinctoria root extracts and their main constituents, hydroxynaphthoquinones. Phytother Res. 2005;19:141–147. - PubMed
-
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. - PubMed
-
- Bogurcu N, Sevimli-Gur C, Ozmen B, Bedir E, Korkmaz KS. ALCAPs induce mitochondrial apoptosis and activate DNA damage response by generating ROS and inhibiting topoisomerase I enzyme activity in K562 leukemia cell line. Biochem Biophys Res Commun. 2011;409:738–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

