Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction
- PMID: 22473777
- PMCID: PMC3424723
- DOI: 10.1113/jphysiol.2011.224485
Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction
Abstract
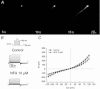
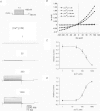
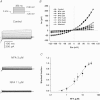
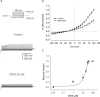
Motility, maturation and the acrosome reaction (AR) are fundamental functions of mammalian spermatozoa. While travelling through the female reproductive tract, spermatozoa must mature through a process named capacitation, so that they can reach the egg and undergo the AR, an exocytotic event necessary to fertilize the egg. Though Cl⁻ is important for sperm capacitation and for the AR, not much is known about the molecular identity of the Cl⁻ transporters involved in these processes.We implemented a modified perforated patch-clamp strategy to obtain whole cell recordings sealing on the head of mature human spermatozoa.Our whole cell recordings revealed the presence of a Ca²⁺-dependent Cl⁻ current. The biophysical characteristics of this current and its sensitivity to niflumic acid (NFA) and 4,4-diisothiocyano-2,2-stilbene disulphonic acid (DIDIS) are consistent with those displayed by the Ca²⁺-dependent Cl⁻ channel from the anoctamin family (TMEM16). Whole cell patch clamp recordings in the cytoplasmic droplet of human spermatozoa corroborated the presence of these currents, which were sensitive to NFA and to a small molecule TMEM16A inhibitor (TMEM16Ainh, an aminophenylthiazole). Importantly, the human sperm AR induced by a recombinant human glycoprotein from the zona pellucida, rhZP3, displayed a similar sensitivity to NFA, DIDS and TMEM16Ainh as the sperm Ca²⁺-dependent Cl⁻ currents. Our findings indicate the presence of Ca²⁺-dependent Cl⁻ currents in human spermatozoa, that TMEM16A may contribute to these currents and also that sperm Ca²⁺-dependent Cl⁻ currents may participate in the rhZP3-induced AR.
Figures


Comment in
-
Chloride channels join the sperm 'channelome'.J Physiol. 2012 Jun 1;590(11):2553-4. doi: 10.1113/jphysiol.2012.234138. J Physiol. 2012. PMID: 22787168 Free PMC article. No abstract available.
References
-
- Arakaki Y, Uehara T, Fagoonee I. Comparative studies of the genus Echinometra from Okinawa and Mauritius. Zoolog Sci. 1998;15:159–168. - PubMed
-
- Axner E, Linde Forsberg C. Sperm morphology in the domestic cat, and its relation with fertility: a retrospective study. Reprod Domest Anim. 2007;42:282–291. - PubMed
-
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56:334–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

