The chemokine CXCL12 and the HIV-1 envelope protein gp120 regulate spontaneous activity of Cajal-Retzius cells in opposite directions
- PMID: 22473778
- PMCID: PMC3406399
- DOI: 10.1113/jphysiol.2011.224873
The chemokine CXCL12 and the HIV-1 envelope protein gp120 regulate spontaneous activity of Cajal-Retzius cells in opposite directions
Abstract
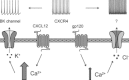
Activation of the CXC chemokine receptor 4 (CXCR4) in Cajal–Retzius cells by CXC chemokine ligand 12 (CXCL12) is important for controlling their excitability. CXCR4 is also a co-receptor for the glycoprotein 120 (gp120) of the envelope of the human immunodeficiency virus type 1 (HIV-1), and binding of gp120 to CXCR4 may produce pathological effects. In order to study CXCR4-dependent modulation of membrane excitability, we recorded in cell-attached configuration spontaneous action currents from hippocampal stratum lacunosum-moleculare Cajal–Retzius cells of the CXCR4-EGFP mouse. CXCL12 (50 nM) powerfully inhibited firing independently of synaptic transmission, suggesting that CXCR4 regulates an intrinsic conductance. This effect was prevented by conditioning slices with BAPTA-AM (200 μM), and by blockers of the BK calcium-dependent potassium channels (TEA (1 mM), paxilline (10 μM) and iberiotoxin (100 nM)). In contrast, exposure to gp120 (pico- to nanomolar range, alone or in combination with soluble cluster of differentiation 4 (CD4)), enhanced spontaneous firing frequency. This effect was prevented by the CXCR4 antagonist AMD3100 (1 μM) and was absent in EGFP-negative stratum lacunosum-moleculare interneurons. Increased excitability was prevented by treating slices with BAPTA-AM or bumetanide, suggesting that gp120 activates a mechanism that is both calcium- and chloride-dependent. In conclusion, our results demonstrate that CXCL12 and gp120 modulate the excitability of Cajal–Retzius cells in opposite directions. We propose that CXCL12 and gp120 either generate calcium responses of different strength or activate distinct pools of intracellular calcium, leading to agonist-specific responses, mediated by BK channels in the case of CXCL12, and by a chloride-dependent mechanism in the case of gp120.
Figures











Comment in
-
Chemokines and HIV-1 virus: opposing players in Cajal-Retzius cell function.J Physiol. 2012 Jul 1;590(13):2949-50. doi: 10.1113/jphysiol.2012.234542. J Physiol. 2012. PMID: 22753620 Free PMC article. No abstract available.
Similar articles
-
Optogenetic activation of cajal-retzius cells reveals their glutamatergic output and a novel feedforward circuit in the developing mouse hippocampus.J Neurosci. 2014 Sep 24;34(39):13018-32. doi: 10.1523/JNEUROSCI.1407-14.2014. J Neurosci. 2014. PMID: 25253849 Free PMC article.
-
Distinctive properties of CXC chemokine receptor 4-expressing Cajal-Retzius cells versus GABAergic interneurons of the postnatal hippocampus.J Physiol. 2010 Aug 1;588(Pt 15):2859-78. doi: 10.1113/jphysiol.2010.190868. Epub 2010 Jun 14. J Physiol. 2010. PMID: 20547684 Free PMC article.
-
Chemokines and HIV-1 virus: opposing players in Cajal-Retzius cell function.J Physiol. 2012 Jul 1;590(13):2949-50. doi: 10.1113/jphysiol.2012.234542. J Physiol. 2012. PMID: 22753620 Free PMC article. No abstract available.
-
Modulation of hippocampal stratum lacunosum-moleculare microcircuits.J Physiol. 2011 Apr 15;589(Pt 8):1885-91. doi: 10.1113/jphysiol.2010.201079. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135043 Free PMC article. Review.
-
Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways.J Leukoc Biol. 2003 Nov;74(5):676-82. doi: 10.1189/jlb.0503206. Epub 2003 Jul 22. J Leukoc Biol. 2003. PMID: 12960231 Review.
Cited by
-
Optogenetic activation of cajal-retzius cells reveals their glutamatergic output and a novel feedforward circuit in the developing mouse hippocampus.J Neurosci. 2014 Sep 24;34(39):13018-32. doi: 10.1523/JNEUROSCI.1407-14.2014. J Neurosci. 2014. PMID: 25253849 Free PMC article.
-
Developmental Profile, Morphology, and Synaptic Connectivity of Cajal-Retzius Cells in the Postnatal Mouse Hippocampus.Cereb Cortex. 2016 Feb;26(2):855-72. doi: 10.1093/cercor/bhv271. Epub 2015 Nov 18. Cereb Cortex. 2016. PMID: 26582498 Free PMC article.
-
Direct interaction of HIV gp120 with neuronal CXCR4 and CCR5 receptors induces cofilin-actin rod pathology via a cellular prion protein- and NOX-dependent mechanism.PLoS One. 2021 Mar 11;16(3):e0248309. doi: 10.1371/journal.pone.0248309. eCollection 2021. PLoS One. 2021. PMID: 33705493 Free PMC article.
-
An Overview of Human Immunodeficiency Virus Type 1-Associated Common Neurological Complications: Does Aging Pose a Challenge?J Alzheimers Dis. 2017;60(s1):S169-S193. doi: 10.3233/JAD-170473. J Alzheimers Dis. 2017. PMID: 28800335 Free PMC article. Review.
-
Experience-Dependent Regulation of Cajal-Retzius Cell Networks in the Developing and Adult Mouse Hippocampus.Cereb Cortex. 2018 Feb 1;28(2):672-687. doi: 10.1093/cercor/bhx153. Cereb Cortex. 2018. PMID: 28637318 Free PMC article.
References
-
- Abraham H, Meyer G. Reelin-expressing neurons in the postnatal and adult human hippocampal formation. Hippocampus. 2003;13:715–727. - PubMed
-
- Abraham H, Toth Z, Seress L. A novel population of calretinin positive neurons comprises reelin-positive Cajal–Retzius cells in the hippocampal formation of the adult domestic pig. Hippocampus. 2004;14:385–401. - PubMed
-
- Ahuja S, Smith SO. Multiple switches in G protein-coupled receptor activation. Trends Pharmacol Sci. 2009;30:494–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

