Modulation of synaptic input by GABAB receptors improves coincidence detection for computation of sound location
- PMID: 22473782
- PMCID: PMC3406390
- DOI: 10.1113/jphysiol.2011.226233
Modulation of synaptic input by GABAB receptors improves coincidence detection for computation of sound location
Abstract
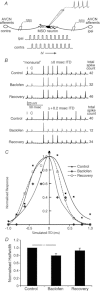
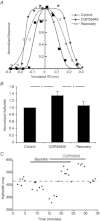
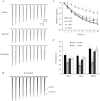
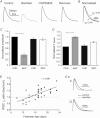
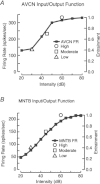
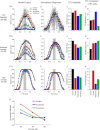
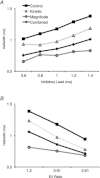
Interaural time disparities (ITDs) are the primary cues for localisation of low-frequency sound stimuli. ITDs are computed by coincidence-detecting neurones in the medial superior olive (MSO) in mammals. Several previous studies suggest that control of synaptic gain is essential for maintaining ITD selectivity as stimulus intensity increases. Using acute brain slices from postnatal day 7 to 24 (P7–P24) Mongolian gerbils, we confirm that activation of GABAB receptors reduces the amplitude of excitatory and inhibitory synaptic currents to the MSO and, moreover, show that the decay kinetics of IPSCs are slowed in mature animals. During repetitive stimuli, activation of GABAB receptors reduced the amount of depression observed, while PSC suppression and the slowed kinetics were maintained. Additionally, we used physiological and modelling approaches to test the potential impact of GABAB activation on ITD encoding in MSO neurones. Current clamp recordings from MSO neurones were made while pharmacologically isolated excitatory inputs were bilaterally stimulated using pulse trains that simulate ITDs in vitro. MSO neurones showed strong selectivity for bilateral delays. Application of both GABAB agonists and antagonists demonstrate that GABAB modulation of synaptic input can sharpen ITD selectivity. We confirmed and extended these results in a computational model that allowed for independent manipulation of each GABAB-dependent effect. Modelling suggests that modulation of both amplitude and kinetics of synaptic inputs by GABAB receptors can improve precision of ITD computation. Our studies suggest that in vivo modulation of synaptic input by GABAB receptors may act to preserve ITD selectivity across various stimulus conditions.
Figures


Comment in
-
GABAB receptors sharpen tuning of a sound localization circuit.J Physiol. 2012 Jul 1;590(13):2951-2. doi: 10.1113/jphysiol.2012.234146. J Physiol. 2012. PMID: 22753621 Free PMC article. No abstract available.
Similar articles
-
The mammalian interaural time difference detection circuit is differentially controlled by GABAB receptors during development.J Neurosci. 2010 Jul 21;30(29):9715-27. doi: 10.1523/JNEUROSCI.1552-10.2010. J Neurosci. 2010. PMID: 20660254 Free PMC article.
-
Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive.J Neurophysiol. 1996 Oct;76(4):2137-56. doi: 10.1152/jn.1996.76.4.2137. J Neurophysiol. 1996. PMID: 8899590
-
Directional hearing by linear summation of binaural inputs at the medial superior olive.Neuron. 2013 Jun 5;78(5):936-48. doi: 10.1016/j.neuron.2013.04.028. Neuron. 2013. PMID: 23764292 Free PMC article.
-
The evolution of temporal processing in the medial superior olive, an auditory brainstem structure.Prog Neurobiol. 2000 Aug;61(6):581-610. doi: 10.1016/s0301-0082(99)00068-4. Prog Neurobiol. 2000. PMID: 10775798 Review.
-
The lateral superior olive: a functional role in sound source localization.Neuroscientist. 2003 Apr;9(2):127-43. doi: 10.1177/1073858403252228. Neuroscientist. 2003. PMID: 12708617 Review.
Cited by
-
GABAB receptors sharpen tuning of a sound localization circuit.J Physiol. 2012 Jul 1;590(13):2951-2. doi: 10.1113/jphysiol.2012.234146. J Physiol. 2012. PMID: 22753621 Free PMC article. No abstract available.
-
Coincidence detection in the medial superior olive: mechanistic implications of an analysis of input spiking patterns.Front Neural Circuits. 2014 May 1;8:42. doi: 10.3389/fncir.2014.00042. eCollection 2014. Front Neural Circuits. 2014. PMID: 24822037 Free PMC article.
-
Adaptation in sound localization: from GABA(B) receptor-mediated synaptic modulation to perception.Nat Neurosci. 2013 Dec;16(12):1840-7. doi: 10.1038/nn.3548. Epub 2013 Oct 20. Nat Neurosci. 2013. PMID: 24141311
-
Physiology and anatomy of neurons in the medial superior olive of the mouse.J Neurophysiol. 2016 Dec 1;116(6):2676-2688. doi: 10.1152/jn.00523.2016. Epub 2016 Sep 21. J Neurophysiol. 2016. PMID: 27655966 Free PMC article.
-
Structure and dynamics that specialize neurons for high-frequency coincidence detection in the barn owl nucleus laminaris.Biol Cybern. 2023 Apr;117(1-2):143-162. doi: 10.1007/s00422-023-00962-z. Epub 2023 May 2. Biol Cybern. 2023. PMID: 37129628
References
-
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. - PubMed
-
- Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABAB receptors. Neuron. 1998;20:135–141. - PubMed
-
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol. 2005;481:6–18. - PubMed
-
- Cant NB, Hyson RL. Projections from the lateral nucleus of the trapezoid body to the medial superior olivary nucleus in the gerbil. Hear Res. 1992;58:26–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

