Aging modulates susceptibility to mouse liver Mallory-Denk body formation
- PMID: 22473941
- PMCID: PMC3393076
- DOI: 10.1369/0022155412441478
Aging modulates susceptibility to mouse liver Mallory-Denk body formation
Abstract
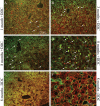
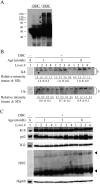
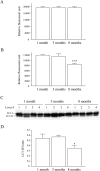
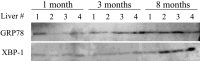
Mallory-Denk bodies (MDBs) are hepatocyte cytoplasmic inclusions found in several liver diseases and consist primarily of the cytoskeletal proteins, keratins 8 and 18 (K8/K18). Recent evidence indicates that the extent of stress-induced protein misfolding, a K8>K18 overexpression state, and transglutaminase-2 activation promote MDB formation. In addition, the genetic background and gender play an important role in mouse MDB formation, but the effect of aging on this process is unknown. Given that oxidative stress increases with aging, the authors hypothesized that aging predisposes to MDB formation. They used an established mouse MDB model-namely, feeding non-transgenic male FVB/N mice (1, 3, and 8 months old) with 3,5 diethoxycarbonyl-1,4-dihydrocollidine for 2 months. MDB formation was assessed using immunofluorescence staining and biochemically by demonstrating keratin and ubiquitin-containing crosslinks generated by transglutaminase-2. Immunofluorescence staining showed that old mice had a significant increase in MDB formation compared with young mice. MDB formation paralleled the generation of high molecular weight ubiquitinated keratin-containing complexes and induction of p62. Old mouse livers had increased oxidative stress. In addition, 20S proteasome activity and autophagy were decreased, and endoplasmic reticulum stress was increased in older livers. Therefore, aging predisposes to experimental MDB formation, possibly by decreased activity of protein degradation machinery.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury.Hepatology. 2008 Sep;48(3):943-52. doi: 10.1002/hep.22436. Hepatology. 2008. PMID: 18697208
-
Oxidative stress, Nrf2 and keratin up-regulation associate with Mallory-Denk body formation in mouse erythropoietic protoporphyria.Hepatology. 2012 Jul;56(1):322-31. doi: 10.1002/hep.25664. Epub 2012 Apr 25. Hepatology. 2012. PMID: 22334478 Free PMC article.
-
CD73 (ecto-5'-nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation.Hepatology. 2013 Nov;58(5):1790-800. doi: 10.1002/hep.26525. Epub 2013 Aug 26. Hepatology. 2013. PMID: 23729294 Free PMC article.
-
Intermediate filament cytoskeleton of the liver in health and disease.Histochem Cell Biol. 2008 Jun;129(6):735-49. doi: 10.1007/s00418-008-0431-x. Epub 2008 Apr 29. Histochem Cell Biol. 2008. PMID: 18443813 Free PMC article. Review.
-
From Mallory to Mallory-Denk bodies: what, how and why?Exp Cell Res. 2007 Jun 10;313(10):2033-49. doi: 10.1016/j.yexcr.2007.04.024. Epub 2007 Apr 27. Exp Cell Res. 2007. PMID: 17531973 Review.
Cited by
-
Keratin 18-deficiency results in steatohepatitis and liver tumors in old mice: A model of steatohepatitis-associated liver carcinogenesis.Oncotarget. 2016 Nov 8;7(45):73309-73322. doi: 10.18632/oncotarget.12325. Oncotarget. 2016. PMID: 27689336 Free PMC article.
-
Mallory-Denk bodies and hepatocellular senescence: a causal relationship?Virchows Arch. 2024 Apr;484(4):637-644. doi: 10.1007/s00428-024-03748-1. Epub 2024 Jan 30. Virchows Arch. 2024. PMID: 38289501 Free PMC article.
-
Methionine and vitamin E supplementation improve production performance, antioxidant potential, and liver health in aged laying hens.Poult Sci. 2024 Dec;103(12):104415. doi: 10.1016/j.psj.2024.104415. Epub 2024 Oct 13. Poult Sci. 2024. PMID: 39488017 Free PMC article.
-
Stable Isotope Labeling Reveals Novel Insights Into Ubiquitin-Mediated Protein Aggregation With Age, Calorie Restriction, and Rapamycin Treatment.J Gerontol A Biol Sci Med Sci. 2018 Apr 17;73(5):561-570. doi: 10.1093/gerona/glx047. J Gerontol A Biol Sci Med Sci. 2018. PMID: 28958078 Free PMC article.
-
Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging.Aging Cell. 2015 Apr;14(2):249-64. doi: 10.1111/acel.12310. Epub 2015 Jan 23. Aging Cell. 2015. PMID: 25620427 Free PMC article.
References
-
- Arteel GE. 2003. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 124:778–790 - PubMed
-
- Bardag-Gorce F, Riley NE, Nan L, Montgomery RO, Li J, French BA, Lue YH, French SW. 2004. The proteasome inhibitor, PS-341, causes cytokeratin aggresome formation. Exp Mol Pathol. 76:9–16 - PubMed
-
- Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. 2008. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 28:13–24 - PubMed
-
- Beckman KB, Ames BN. 1998. The free radical theory of aging matures. Physiol Rev. 78:547–581 - PubMed
-
- Brunt EM. 2010. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 7:195–203 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

