The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease
- PMID: 22473957
- PMCID: PMC3328361
- DOI: 10.1084/jem.20112285
The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease
Abstract
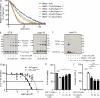
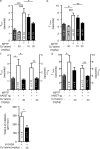
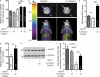
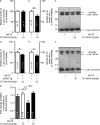
Parkinson's disease (PD) is a progressive, chronic disease characterized by dyskinesia, rigidity, instability, and tremors. The disease is defined by the presence of Lewy bodies, which primarily consist of aggregated α-synuclein protein, and is accompanied by the loss of monoaminergic neurons. Current therapeutic strategies only give symptomatic relief of motor impairment and do not address the underlying neurodegeneration. Hence, we have identified Cu(II)(atsm) as a potential therapeutic for PD. Drug administration to four different animal models of PD resulted in improved motor and cognition function, rescued nigral cell loss, and improved dopamine metabolism. In vitro, this compound is able to inhibit the effects of peroxynitrite-driven toxicity, including the formation of nitrated α-synuclein oligomers. Our results show that Cu(II)(atsm) is effective in reversing parkinsonian defects in animal models and has the potential to be a successful treatment of PD.
Figures


References
-
- Andreadou E., Nikolaou C., Gournaras F., Rentzos M., Boufidou F., Tsoutsou A., Zournas C., Zissimopoulos V., Vassilopoulos D. 2009. Serum uric acid levels in patients with Parkinson’s disease: their relationship to treatment and disease duration. Clin. Neurol. Neurosurg. 111:724–728 10.1016/j.clineuro.2009.06.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

