ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation
- PMID: 22473958
- PMCID: PMC3328364
- DOI: 10.1084/jem.20111202
ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation
Abstract
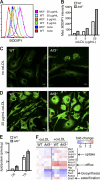
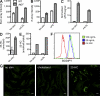
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipid-loaded macrophages in the arterial wall. We demonstrate that macrophage lipid body formation can be induced by modified lipoproteins or by inflammatory Toll-like receptor agonists. We used an unbiased approach to study the overlap in these pathways to identify regulators that control foam cell formation and atherogenesis. An analysis method integrating epigenomic and transcriptomic datasets with a transcription factor (TF) binding site prediction algorithm suggested that the TF ATF3 may regulate macrophage foam cell formation. Indeed, we found that deletion of this TF results in increased lipid body accumulation, and that ATF3 directly regulates transcription of the gene encoding cholesterol 25-hydroxylase. We further showed that production of 25-hydroxycholesterol (25-HC) promotes macrophage foam cell formation. Finally, deletion of ATF3 in Apoe(-/-) mice led to in vivo increases in foam cell formation, aortic 25-HC levels, and disease progression. These results define a previously unknown role for ATF3 in controlling macrophage lipid metabolism and demonstrate that ATF3 is a key intersection point for lipid metabolic and inflammatory pathways in these cells.
Figures



References
-
- Bauman D.R., Bitmansour A.D., McDonald J.G., Thompson B.M., Liang G., Russell D.W. 2009. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA. 106:16764–16769 10.1073/pnas.0909142106 - DOI - PMC - PubMed
-
- Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J.R. Stat. Soc. Ser. B. 57:289–300
-
- Brown M.S., Dana S.E., Goldstein J.L. 1975. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J. Biol. Chem. 250:4025–4027 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

