Polar auxin transport: an early invention
- PMID: 22473986
- PMCID: PMC3398450
- DOI: 10.1093/jxb/ers106
Polar auxin transport: an early invention
Abstract
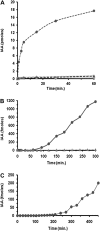
In higher plants, cell-to-cell polar auxin transport (PAT) of the phytohormone auxin, indole-3-acetic acid (IAA), generates maxima and minima that direct growth and development. Although IAA is present in all plant phyla, PAT has only been detected in land plants, the earliest being the Bryophytes. Charophyta, a group of freshwater green algae, are among the first multicellular algae with a land plant-like phenotype and are ancestors to land plants. IAA has been detected in members of Charophyta, but its developmental role and the occurrence of PAT are unknown. We show that naphthylphthalamic acid (NPA)-sensitive PAT occurs in internodal cells of Chara corallina. The relatively high velocity (at least 4-5 cm/h) of auxin transport through the giant (3-5 cm) Chara cells does not occur by simple diffusion and is not sensitive to a specific cytoplasmic streaming inhibitor. The results demonstrate that PAT evolved early in multicellular plant life. The giant Chara cells provide a unique new model system to study PAT, as Chara allows the combining of real-time measurements and mathematical modelling with molecular, developmental, cellular, and electrophysiological studies.
Figures


Comment in
-
Polar auxin transport in relation to long-distance transport of nutrients in the Charales.J Exp Bot. 2013 Jan;64(1):1-9. doi: 10.1093/jxb/ers358. J Exp Bot. 2013. PMID: 23264638
References
-
- Berecki G, Varga Z, van Iren F, van Duijn B. Anion channels in Chara corallina tonoplast membrane: calcium dependence and rectification. Journal of Membrane Biology. 1999;172:159–168. - PubMed
-
- Boutte Y, Ikeda Y, Grebe M. Mechanisms of auxin-dependent cell and tissue polarity. Current Opinion in Plant Biology. 2007;10:616–623. - PubMed
-
- Cande WZ, Goldsmith MHM, Ray PM. Polar auxin transport and auxin-induced elongation in the absence of cytoplasmic streaming. Planta. 1973;111:279–296. - PubMed
-
- Cooke TJ, Poli DB, Sztein AE, Cohen JD. Evolutionary patterns in auxin action. Plant Molecular Biology. 2002;49:319–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

