NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKα activation
- PMID: 22474019
- PMCID: PMC3532048
- DOI: 10.4049/jimmunol.1200021
NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKα activation
Abstract
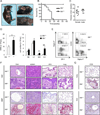
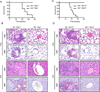
Immune cell-mediated tissue injury is a common feature of different inflammatory diseases, yet the pathogenetic mechanisms and cell types involved vary significantly. Hypereosinophilic syndrome (HES) represents a group of inflammatory diseases that is characterized by increased numbers of pathogenic eosinophilic granulocytes in the peripheral blood and diverse organs. On the basis of clinical and laboratory findings, various forms of HES have been defined, yet the molecular mechanism and potential signaling pathways that drive eosinophil expansion remain largely unknown. In this study, we show that mice deficient of the serine/threonine-specific protein kinase NF-κB-inducing kinase (NIK) develop a HES-like disease, reflected by progressive blood and tissue eosinophilia, tissue injury, and premature death at around 25-30 wk of age. Similar to the lymphocytic form of HES, CD4(+) T cells from NIK-deficient mice express increased levels of Th2-associated cytokines, and eosinophilia and survival of NIK-deficient mice could be prevented completely by genetic ablation of CD4(+) T cells. Experiments based on bone marrow chimeric mice, however, demonstrated that inflammation in NIK-deficient mice depended on radiation-resistant tissues, implicating that NIK-deficient immune cells mediate inflammation in a nonautonomous manner. Surprisingly, disease development was independent of NIK's known function as an IκB kinase α (IKKα) kinase, because mice carrying a mutation in the activation loop of IKKα, which is phosphorylated by NIK, did not develop inflammatory disease. Our data show that NIK activity in nonhematopoietic cells controls Th2 cell development and prevents eosinophil-driven inflammatory disease, most likely using a signaling pathway that operates independent of the known NIK substrate IKKα.
Figures



References
-
- Gotlib J. World Health Organization-defined eosinophilic disorders: 2011 update on diagnosis, risk stratification, and management. Am. J. Hematol. 86:677–688. - PubMed
-
- Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, Nutman TB, Pfab F, Ring J, Rothenberg ME, Roufosse F, Sajous MH, Sheikh J, Simon D, Simon HU, Stein ML, Wardlaw A, Weller PF, Klion AD. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 2009;124:1319–1325. e1313. - PMC - PubMed
-
- Gleich GJ, Leiferman KM. The hypereosinophilic syndromes: current concepts and treatments. Br. J. Haematol. 2009;145:271–285. - PubMed
-
- Roufosse F, Cogan E, Goldman M. Recent advances in pathogenesis and management of hypereosinophilic syndromes. Allergy. 2004;59:673–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

