Effects of CETP inhibition on triglyceride-rich lipoprotein composition and apoB-48 metabolism
- PMID: 22474066
- PMCID: PMC3351826
- DOI: 10.1194/jlr.M019570
Effects of CETP inhibition on triglyceride-rich lipoprotein composition and apoB-48 metabolism
Abstract
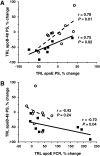
Cholesteryl ester transfer protein (CETP) facilitates the transfer of HDL cholesteryl ester to triglyceride-rich lipoproteins (TRL). This study aimed to determine the effects of CETP inhibition with torcetrapib on TRL composition and apoB-48 metabolism. Study subjects with low HDL cholesterol (<40 mg/dl), either untreated (n = 9) or receiving atorvastatin 20 mg daily (n = 9), received placebo for 4 weeks, followed by torcetrapib 120 mg once daily for the next 4 weeks. A subset of the subjects not treated with atorvastatin participated in a third phase (n = 6), in which they received torcetrapib 120 mg twice daily for an additional 4 weeks. At the end of each phase, all subjects received a primed-constant infusion of [5,5,5-(2)H(3)]L-leucine, while in the constantly fed state, to determine the kinetics of TRL apoB-48 and TRL composition. Relative to placebo, torcetrapib markedly reduced TRL CE levels in all groups (≥-69%; P < 0.005). ApoB-48 pool size (PS) and production rate (PR) decreased in the nonatorvastatin once daily (PS: -49%, P = 0.007; PR: -49%, P = 0.005) and twice daily (PS: -30%, P = 0.01; PR: -27%, P = 0.13) cohorts. In the atorvastatin cohort, apoB-48 PS and PR, which were already lowered by atorvastatin, did not change with torcetrapib. Our findings indicate that CETP inhibition reduced plasma apoB-48 concentrations by reducing apoB-48 production but did not have this effect in subjects already treated with atorvastatin.
Figures

Comment in
-
The inhibition of cholesteryl ester transfer protein: a long and winding road.J Lipid Res. 2012 Jun;53(6):1039-41. doi: 10.1194/jlr.E027334. Epub 2012 Apr 10. J Lipid Res. 2012. PMID: 22496386 Free PMC article. No abstract available.
References
-
- Zilversmit D. B. 1995. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich-remnant lipoproteins. Clin. Chem. 41: 153–158 - PubMed
-
- Karpe F. 1999. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 246: 341–355 - PubMed
-
- Patsch J. R., Miesenböck G., Hopferwieser T., Mühlberger V., Knapp E., Dunn J. K., Gotto A. M., Jr, Patsch W. 1992. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. 12: 1336–1345 - PubMed
-
- Proctor S. D., Vine D. F., Mamo J. C. L. 2002. Arterial retention of apolipoprotein B48- and B100-containing lipoproteins in atherogenesis. Curr. Opin. Lipidol. 13: 461–470 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

