The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women
- PMID: 22474224
- PMCID: PMC3334365
- DOI: 10.1093/cid/cis225
The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women
Abstract
Background: Recent trials report the short-term efficacy of tenofovir-based pre-exposure prophylaxis (PrEP) for prevention of human immunodeficiency virus (HIV) infection. PrEP's long-term impact on patient outcomes, population-level transmission, and cost-effectiveness remains unknown.
Methods: We linked data from recent trials to a computer model of HIV acquisition, screening, and care to project lifetime HIV risk, life expectancy (LE), costs, and cost-effectiveness, using 2 PrEP-related strategies among heterosexual South African women: (1) women receiving no PrEP and (2) women not receiving PrEP (a tenofovir-based vaginal microbicide). We used a South African clinical cohort and published data to estimate population demographic characteristics, age-adjusted incidence of HIV infection, and HIV natural history and treatment parameters. Baseline PrEP efficacy (percentage reduction in HIV transmission) was 39% at a monthly cost of $5 per woman. Alternative parameter values were examined in sensitivity analyses.
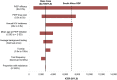
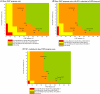
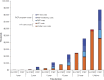
Results: Among South African women, PrEP reduced mean lifetime HIV risk from 40% to 27% and increased population discounted (undiscounted) LE from 22.51 (41.66) to 23.48 (44.48) years. Lifetime costs of care increased from $7280 to $9890 per woman, resulting in an incremental cost-effectiveness ratio of $2700/year of life saved, and may, under optimistic assumptions, achieve cost savings. Under baseline HIV infection incidence assumptions, PrEP was not cost saving, even assuming an efficacy >60% and a cost <$1. At an HIV infection incidence of 9.1%/year, PrEP achieved cost savings at efficacies ≥50%.
Conclusions: PrEP in South African women is very cost-effective by South African standards, conferring excellent value under virtually all plausible data scenarios. Although optimistic assumptions would be required to achieve cost savings, these represent important benchmarks for future PrEP study design.
Figures
Similar articles
-
HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness.Clin Infect Dis. 2009 Mar 15;48(6):806-15. doi: 10.1086/597095. Clin Infect Dis. 2009. PMID: 19193111 Free PMC article.
-
Potential Clinical and Economic Value of Long-Acting Preexposure Prophylaxis for South African Women at High-Risk for HIV Infection.J Infect Dis. 2016 May 15;213(10):1523-31. doi: 10.1093/infdis/jiv523. Epub 2015 Dec 17. J Infect Dis. 2016. PMID: 26681778 Free PMC article.
-
The cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men and transgender women at high risk of HIV infection in Brazil.J Int AIDS Soc. 2018 Mar;21(3):e25096. doi: 10.1002/jia2.25096. J Int AIDS Soc. 2018. PMID: 29603888 Free PMC article.
-
Cost-effectiveness of pre-exposure prophylaxis for HIV: a review.Curr Opin HIV AIDS. 2012 Nov;7(6):587-92. doi: 10.1097/COH.0b013e3283582c8b. Curr Opin HIV AIDS. 2012. PMID: 23076124 Review.
-
Where to deploy pre-exposure prophylaxis (PrEP) in sub-Saharan Africa?Sex Transm Infect. 2013 Dec;89(8):628-34. doi: 10.1136/sextrans-2012-050891. Epub 2013 Aug 2. Sex Transm Infect. 2013. PMID: 23912819 Review.
Cited by
-
Reproductive and maternal healthcare needs of HIV infected women.Curr HIV/AIDS Rep. 2013 Dec;10(4):333-41. doi: 10.1007/s11904-013-0172-x. Curr HIV/AIDS Rep. 2013. PMID: 23918674 Review.
-
Use of antiretrovirals for HIV prevention: what do we know and what don't we know?Curr HIV/AIDS Rep. 2013 Jun;10(2):142-51. doi: 10.1007/s11904-013-0157-9. Curr HIV/AIDS Rep. 2013. PMID: 23494772 Free PMC article. Review.
-
Engaging healthcare providers to implement HIV pre-exposure prophylaxis.Curr Opin HIV AIDS. 2012 Nov;7(6):593-9. doi: 10.1097/COH.0b013e3283590446. Curr Opin HIV AIDS. 2012. PMID: 23032736 Free PMC article. Review.
-
Risks and benefits of oral HIV pre-exposure prophylaxis for people with chronic hepatitis B.Lancet HIV. 2022 Aug;9(8):e585-e594. doi: 10.1016/S2352-3018(22)00123-0. Epub 2022 Jul 8. Lancet HIV. 2022. PMID: 35817068 Free PMC article. Review.
-
HIV-1 self-testing to improve the efficiency of pre-exposure prophylaxis delivery: a randomized trial in Kenya.Trials. 2019 Jul 4;20(1):396. doi: 10.1186/s13063-019-3521-2. Trials. 2019. PMID: 31272495 Free PMC article.
References
-
- Thigpen MC, Kebaabetswe PM, Smith DK, et al. 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention.: Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study [oral abstract WELBC01] Rome, Italy. 2011.
-
- Baeten J. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP study [oral abstract MOAX0106] 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention. Rome, Italy. 2011.
-
- Family Health International. FHI to initiate orderly closure of FEM-PrEP. Available at: http://www.fhi.org/en/Research/Projects/FEM-PrEP.htm. Accessed 24 May 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

