Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis
- PMID: 22474281
- PMCID: PMC3366851
- DOI: 10.1074/jbc.M112.359950
Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis
Abstract
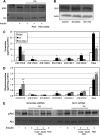
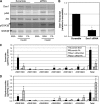
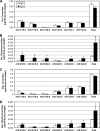
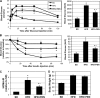
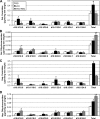
Fenretinide is a synthetic retinoid that is being tested in clinical trials for the treatment of breast cancer and insulin resistance, but its mechanism of action has been elusive. Recent in vitro data indicate that fenretinide inhibits dihydroceramide desaturase, an enzyme involved in the biosynthesis of lipotoxic ceramides that antagonize insulin action. Because of this finding, we assessed whether fenretinide could improve insulin sensitivity and glucose homeostasis in vitro and in vivo by controlling ceramide production. The effect of fenretinide on insulin action and the cellular lipidome was assessed in a number of lipid-challenged models including cultured myotubes and isolated muscles strips incubated with exogenous fatty acids and mice fed a high-fat diet. Insulin action was evaluated in the various models by measuring glucose uptake or disposal and the activation of Akt/PKB, a serine/threonine kinase that is obligate for insulin-stimulated anabolism. The effects of fenretinide on cellular lipid levels were assessed by LC-MS/MS. Fenretinide negated lipid-induced insulin resistance in each of the model systems assayed. Simultaneously, the drug depleted cells of ceramide, while promoting the accumulation of the precursor dihydroceramide, a substrate for the reaction catalyzed by Des1. These data suggest that fenretinide improves insulin sensitivity, at least in part, by inhibiting Des1 and suggest that therapeutics targeting this enzyme may be a viable therapeutic means for normalizing glucose homeostasis in the overweight and diabetic.
Figures



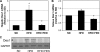
Similar articles
-
Fenretinide inhibits obesity and fatty liver disease but induces Smpd3 to increase serum ceramides and worsen atherosclerosis in LDLR-/- mice.Sci Rep. 2023 Mar 9;13(1):3937. doi: 10.1038/s41598-023-30759-w. Sci Rep. 2023. PMID: 36894641 Free PMC article.
-
Sulforaphane Prevents Hepatic Insulin Resistance by Blocking Serine Palmitoyltransferase 3-Mediated Ceramide Biosynthesis.Nutrients. 2019 May 27;11(5):1185. doi: 10.3390/nu11051185. Nutrients. 2019. PMID: 31137828 Free PMC article.
-
Fenretinide ameliorates insulin resistance and fatty liver in obese mice.Biol Pharm Bull. 2012;35(3):369-75. doi: 10.1248/bpb.35.369. Biol Pharm Bull. 2012. PMID: 22382323
-
The mechanisms of Fenretinide-mediated anti-cancer activity and prevention of obesity and type-2 diabetes.Biochem Pharmacol. 2014 Oct 1;91(3):277-86. doi: 10.1016/j.bcp.2014.07.012. Epub 2014 Jul 25. Biochem Pharmacol. 2014. PMID: 25069047 Review.
-
Mechanism of fenretinide (4-HPR)-induced cell death.Apoptosis. 2001 Oct;6(5):377-88. doi: 10.1023/a:1011342220621. Apoptosis. 2001. PMID: 11483862 Review.
Cited by
-
Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide.Rev Endocr Metab Disord. 2013 Mar;14(1):59-68. doi: 10.1007/s11154-012-9235-3. Rev Endocr Metab Disord. 2013. PMID: 23292334 Free PMC article. Review.
-
Ablation of dihydroceramide desaturase confers resistance to etoposide-induced apoptosis in vitro.PLoS One. 2012;7(9):e44042. doi: 10.1371/journal.pone.0044042. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984457 Free PMC article.
-
Emerging Roles for Sphingolipids in Cardiometabolic Disease: A Rational Therapeutic Target?Nutrients. 2024 Sep 28;16(19):3296. doi: 10.3390/nu16193296. Nutrients. 2024. PMID: 39408263 Free PMC article. Review.
-
Contribution of specific ceramides to obesity-associated metabolic diseases.Cell Mol Life Sci. 2022 Jul 5;79(8):395. doi: 10.1007/s00018-022-04401-3. Cell Mol Life Sci. 2022. PMID: 35789435 Free PMC article. Review.
-
Intramyocellular ceramides and skeletal muscle mitochondrial respiration are partially regulated by Toll-like receptor 4 during hindlimb unloading.Am J Physiol Regul Integr Comp Physiol. 2016 Nov 1;311(5):R879-R887. doi: 10.1152/ajpregu.00253.2016. Epub 2016 Aug 31. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27581814 Free PMC article.
References
-
- Antonoff M. B., D'Cunha J. (2010) Teaching an old drug new tricks: metformin as a targeted therapy for lung cancer. Semin. Thorac. Cardiovasc. Surg. 22, 195–196 - PubMed
-
- Heemskerk J., Tobin A. J., Bain L. J. (2002) Teaching old drugs new tricks: Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA. Trends Neurosci. 25, 494–496 - PubMed
-
- Bonanni B., Lazzeroni M. (2009) Retinoids and breast cancer prevention. Recent Results Cancer Res. 181, 77–82 - PubMed
-
- Wang H., Charles A. G., Frankel A. J., Cabot M. C. (2003) Increasing intracellular ceramide: an approach that enhances the cytotoxic response in prostate cancer cells. Urology 61, 1047–1052 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

