Interaction of Notch signaling modulator Numb with α-Adaptin regulates endocytosis of Notch pathway components and cell fate determination of neural stem cells
- PMID: 22474327
- PMCID: PMC3366835
- DOI: 10.1074/jbc.M112.360719
Interaction of Notch signaling modulator Numb with α-Adaptin regulates endocytosis of Notch pathway components and cell fate determination of neural stem cells
Abstract
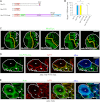
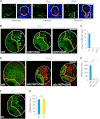
The ability to balance self-renewal and differentiation is a hallmark of stem cells. In Drosophila neural stem cells (NSCs), Numb/Notch (N) signaling plays a key role in this process. However, the molecular and cellular mechanisms underlying Numb function in a stem cell setting remain poorly defined. Here we show that α-Adaptin (α-Ada), a subunit of the endocytic AP-2 complex, interacts with Numb through a new mode of interaction to regulate NSC homeostasis. In α-ada mutants, N pathway component Sanpodo and the N receptor itself exhibited altered trafficking, and N signaling was up-regulated in the intermediate progenitors of type II NSC lineages, leading to their transformation into ectopic NSCs. Surprisingly, although the Ear domain of α-Ada interacts with the C terminus of Numb and is important for α-Ada function in the sensory organ precursor lineage, it was dispensable in the NSCs. Instead, α-Ada could regulate Sanpodo, N trafficking, and NSC homeostasis by interacting with Numb through new domains in both proteins previously not known to mediate their interaction. This interaction could be bypassed when α-Ada was directly fused to the phospho-tyrosine binding domain of Numb. Our results identify a critical role for the AP-2-mediated endocytosis in regulating NSC behavior and reveal a new mechanism by which Numb regulates NSC behavior through N. These findings are likely to have important implications for cancer biology.
Figures

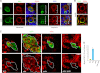
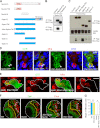
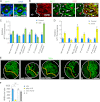
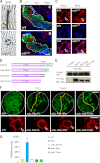
Similar articles
-
Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs.EMBO Rep. 2005 Sep;6(9):836-42. doi: 10.1038/sj.embor.7400500. EMBO Rep. 2005. PMID: 16113648 Free PMC article.
-
Numb inhibits the recycling of Sanpodo in Drosophila sensory organ precursor.Curr Biol. 2013 Apr 8;23(7):581-7. doi: 10.1016/j.cub.2013.02.020. Epub 2013 Mar 21. Curr Biol. 2013. PMID: 23523246
-
Numb regulates the balance between Notch recycling and late-endosome targeting in Drosophila neural progenitor cells.Mol Biol Cell. 2016 Sep 15;27(18):2857-66. doi: 10.1091/mbc.E15-11-0751. Epub 2016 Jul 27. Mol Biol Cell. 2016. PMID: 27466320 Free PMC article.
-
Endocytosis and control of Notch signaling.Curr Opin Cell Biol. 2012 Aug;24(4):534-40. doi: 10.1016/j.ceb.2012.06.006. Epub 2012 Jul 18. Curr Opin Cell Biol. 2012. PMID: 22818956 Free PMC article. Review.
-
The Multitasker Protein: A Look at the Multiple Capabilities of NUMB.Cells. 2023 Jan 15;12(2):333. doi: 10.3390/cells12020333. Cells. 2023. PMID: 36672267 Free PMC article. Review.
Cited by
-
Kin17 regulates proper cortical localization of Miranda in Drosophila neuroblasts by regulating Flfl expression.Cell Rep. 2024 Mar 26;43(3):113823. doi: 10.1016/j.celrep.2024.113823. Epub 2024 Feb 21. Cell Rep. 2024. PMID: 38386552 Free PMC article.
-
Enhanced effect of combining bone marrow mesenchymal stem cells (BMMSCs) and pulsed electromagnetic fields (PEMF) to promote recovery after spinal cord injury in mice.MedComm (2020). 2022 Aug 3;3(3):e160. doi: 10.1002/mco2.160. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35949547 Free PMC article.
-
Loss of nuclear NOTCH1, but not its negative regulator NUMB, is an independent predictor of cervical malignancy.Oncotarget. 2018 Apr 10;9(27):18916-18928. doi: 10.18632/oncotarget.24828. eCollection 2018 Apr 10. Oncotarget. 2018. PMID: 29721172 Free PMC article.
-
Histidine is selectively required for the growth of Myc-dependent dedifferentiation tumours in the Drosophila CNS.EMBO J. 2019 Apr 1;38(7):e99895. doi: 10.15252/embj.201899895. Epub 2019 Feb 25. EMBO J. 2019. PMID: 30804004 Free PMC article.
-
A fluorescent tagging approach in Drosophila reveals late endosomal trafficking of Notch and Sanpodo.J Cell Biol. 2014 Nov 10;207(3):351-63. doi: 10.1083/jcb.201407071. Epub 2014 Nov 3. J Cell Biol. 2014. PMID: 25365996 Free PMC article.
References
-
- Varnum-Finney B., Xu L., Brashem-Stein C., Nourigat C., Flowers D., Bakkour S., Pear W. S., Bernstein I. D. (2000) Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 6, 1278–1281 - PubMed
-
- Mizutani K., Yoon K., Dang L., Tokunaga A., Gaiano N. (2007) Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 - PubMed
-
- Ohlstein B., Spradling A. (2007) Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science 315, 988–992 - PubMed
-
- Luo D., Renault V. M., Rando T. A. (2005) The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 16, 612–622 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

