O-linked N,N'-diacetyllactosamine (LacdiNAc)-modified glycans in extracellular matrix glycoproteins are specifically phosphorylated at subterminal N-acetylglucosamine
- PMID: 22474328
- PMCID: PMC3365741
- DOI: 10.1074/jbc.M111.280297
O-linked N,N'-diacetyllactosamine (LacdiNAc)-modified glycans in extracellular matrix glycoproteins are specifically phosphorylated at subterminal N-acetylglucosamine
Abstract
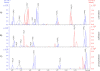
The terminal modification of glycans by β4 addition of N-acetylgalactosamine to N-acetylglucosamine with formation of the N,N-diacetyllactosediamine (LacdiNAc) moiety has been well documented for a number of N-linked glycoproteins and peptides, like neurohormones. Much less is known about O-glycoproteins in this regard because only human zona pellucida glycoprotein 3 (ZP3) and bovine proopiomelanocortin were reported to be LacdiNAc-modified. In searching for mammalian proteins modified with O-linked LacdiNAc we identified six positive species among nine endogenous and recombinant O-glycoproteins, which were extracellular matrix, or matrix-related proteins. These are ZP3 and the five novel LacdiNAc-positive species ECM1, AMACO, nidogen-1, α-dystroglycan, and neurofascin. The mass spectrometric analyses revealed a core 2-based tetrasaccharide as the common structural basis of O-linked LacdiNAc that could be further modified, similar to the type 2 LacNAc termini, with fucose, sialic acid, or sulfate. Here, we provide structural evidence for a novel type of mucin-type O-glycans that is strictly specific for LacdiNAc termini: sugar phosphorylation with formation of GalNAcβ1-4(phospho-)GlcNAc. The structural details of the phosphatase-labile compound were elucidated by MS(2) analysis of tetralysine complexes and by MS(n) measurements of the permethylated glycan alditols. Phospho-LacdiNAc was detected in human HEK-293 as well as in mouse myoblast cells and in bovine brain tissue.
Figures


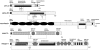




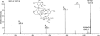
Similar articles
-
Characterization of the acidic N-linked glycans of the zona pellucida of prepuberal pigs by a mass spectrometric approach.Carbohydr Res. 2009 Aug 17;344(12):1541-9. doi: 10.1016/j.carres.2009.05.002. Epub 2009 May 8. Carbohydr Res. 2009. PMID: 19524219
-
Lewis X-containing glycans are specific and potent competitive inhibitors of the binding of ZP3 to complementary sites on capacitated, acrosome-intact mouse sperm.Biol Reprod. 2004 Sep;71(3):770-7. doi: 10.1095/biolreprod.103.023812. Epub 2004 May 5. Biol Reprod. 2004. PMID: 15128590
-
Novel poly-GalNAcbeta1-4GlcNAc (LacdiNAc) and fucosylated poly-LacdiNAc N-glycans from mammalian cells expressing beta1,4-N-acetylgalactosaminyltransferase and alpha1,3-fucosyltransferase.J Biol Chem. 2005 Apr 1;280(13):12810-9. doi: 10.1074/jbc.M414273200. Epub 2005 Jan 14. J Biol Chem. 2005. PMID: 15653684
-
Biosynthesis and Biological Significances of LacdiNAc Group on N- and O-Glycans in Human Cancer Cells.Biomolecules. 2022 Jan 24;12(2):195. doi: 10.3390/biom12020195. Biomolecules. 2022. PMID: 35204696 Free PMC article. Review.
-
Structural and functional attributes of zona pellucida glycoproteins.Soc Reprod Fertil Suppl. 2007;63:203-16. Soc Reprod Fertil Suppl. 2007. PMID: 17566274 Review.
Cited by
-
Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3.Bioengineering (Basel). 2017 Apr 5;4(2):31. doi: 10.3390/bioengineering4020031. Bioengineering (Basel). 2017. PMID: 28952509 Free PMC article.
-
Polysialic acid on neuropilin-2 is exclusively synthesized by the polysialyltransferase ST8SiaIV and attached to mucin-type o-glycans located between the b2 and c domain.J Biol Chem. 2013 Aug 9;288(32):22880-92. doi: 10.1074/jbc.M113.463927. Epub 2013 Jun 25. J Biol Chem. 2013. PMID: 23801331 Free PMC article.
-
Trefoil factor family domains represent highly efficient conformational determinants for N-linked N,N'-di-N-acetyllactosediamine (LacdiNAc) synthesis.J Biol Chem. 2014 Oct 24;289(43):29677-90. doi: 10.1074/jbc.M114.596049. Epub 2014 Sep 10. J Biol Chem. 2014. PMID: 25210040 Free PMC article.
-
Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis.Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15723-8. doi: 10.1073/pnas.1417993111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331875 Free PMC article.
-
Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori.J Biol Chem. 2014 Oct 3;289(40):27363-75. doi: 10.1074/jbc.M114.597757. Epub 2014 Aug 14. J Biol Chem. 2014. PMID: 25124036 Free PMC article.
References
-
- Manzella S. M., Hooper L. V., Baenziger J. U. (1996) Oligosaccharides containing β-1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 271, 12117–12120 - PubMed
-
- Lowe J. B., Marth J. D. (2003) A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72, 643–691 - PubMed
-
- Green E. D., van Halbeek H., Boime I., Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 - PubMed
-
- Dell A., Morris H. R., Easton R. L., Panico M., Patankar M., Oehniger S., Koistinen R., Koistinen H., Seppala M., Clark G. F. (1995) Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J. Biol. Chem. 270, 24116–24126 - PubMed
-
- Manzella S. M., Dharmesh S. M., Cohick C. B., Soares M. J., Baenziger J. U. (1997) Developmental regulation of a pregnancy-specific oligosaccharide structure, NeuAcα2,6GalNAcβ1,4GlcNAc, on select members of the rat placental prolactin family. J. Biol. Chem. 272, 4775–4782 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

