Strategies to reverse endothelial progenitor cell dysfunction in diabetes
- PMID: 22474422
- PMCID: PMC3296202
- DOI: 10.1155/2012/471823
Strategies to reverse endothelial progenitor cell dysfunction in diabetes
Abstract
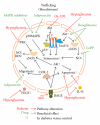
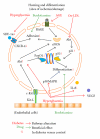
Bone-marrow-derived cells-mediated postnatal vasculogenesis has been reported as the main responsible for the regulation of vascular homeostasis in adults. Since their discovery, endothelial progenitor cells have been depicted as mediators of postnatal vasculogenesis for their peculiar phenotype (partially staminal and partially endothelial), their ability to differentiate in endothelial cell line and to be incorporated into the vessels wall during ischemia/damage. Diabetes mellitus, a condition characterized by cardiovascular disease, nephropathy, and micro- and macroangiopathy, showed a dysfunction of endothelial progenitor cells. Herein, we review the mechanisms involved in diabetes-related dysfunction of endothelial progenitor cells, highlighting how hyperglycemia affects the different steps of endothelial progenitor cells lifetime (i.e., bone marrow mobilization, trafficking into the bloodstream, differentiation in endothelial cells, and homing in damaged tissues/organs). Finally, we review preclinical and clinical strategies that aim to revert diabetes-induced dysfunction of endothelial progenitor cells as a means of finding new strategies to prevent diabetic complications.
Figures

Similar articles
-
Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function.Arterioscler Thromb Vasc Biol. 2014 Jun;34(6):1136-43. doi: 10.1161/ATVBAHA.114.302192. Epub 2014 Apr 17. Arterioscler Thromb Vasc Biol. 2014. PMID: 24743430 Review.
-
Progenitor cells in vascular repair.Curr Opin Lipidol. 2007 Oct;18(5):534-9. doi: 10.1097/MOL.0b013e3282a66082. Curr Opin Lipidol. 2007. PMID: 17885424 Review.
-
The effects of flap ischemia on normal and diabetic progenitor cell function.Plast Reconstr Surg. 2008 Jun;121(6):1929-1942. doi: 10.1097/PRS.0b013e3181715218. Plast Reconstr Surg. 2008. PMID: 18520878
-
Exercise has a positive effect on endothelial progenitor cells, which could be necessary for vascular adaptation processes.Int J Sports Med. 2007 May;28(5):374-80. doi: 10.1055/s-2006-924364. Epub 2006 Nov 16. Int J Sports Med. 2007. PMID: 17111312 Review.
-
Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus.J Cell Mol Med. 2009 Sep;13(9B):3405-14. doi: 10.1111/j.1582-4934.2009.00655.x. J Cell Mol Med. 2009. PMID: 20196780 Free PMC article.
Cited by
-
Hypoxic Preconditioning Enhances Biological Function of Endothelial Progenitor Cells via Notch-Jagged1 Signaling Pathway.Med Sci Monit. 2017 Sep 29;23:4665-4667. doi: 10.12659/msm.902470. Med Sci Monit. 2017. PMID: 28959004 Free PMC article.
-
Mononuclear cell therapy reverts cuff-induced thrombosis in apolipoprotein E-deficient mice.Lipids Health Dis. 2012 Jul 31;11:96. doi: 10.1186/1476-511X-11-96. Lipids Health Dis. 2012. PMID: 22849299 Free PMC article.
-
It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications.Exp Diabetes Res. 2012;2012:742976. doi: 10.1155/2012/742976. Epub 2012 Apr 3. Exp Diabetes Res. 2012. PMID: 22548049 Free PMC article. Review.
-
Interleukin-1β augments the angiogenesis of endothelial progenitor cells in an NF-κB/CXCR7-dependent manner.J Cell Mol Med. 2020 May;24(10):5605-5614. doi: 10.1111/jcmm.15220. Epub 2020 Apr 2. J Cell Mol Med. 2020. PMID: 32239650 Free PMC article.
-
Elucidation of endothelial progenitor cell dysfunction in diabetes by RNA sequencing and constructing lncRNA-miRNA-mRNA competing endogenous RNA network.J Mol Med (Berl). 2022 Nov;100(11):1569-1585. doi: 10.1007/s00109-022-02251-x. Epub 2022 Sep 12. J Mol Med (Berl). 2022. PMID: 36094536
References
-
- Fadini GP, Agostini C, Sartore S, Avogaro A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis. 2007;194(1):46–54. - PubMed
-
- Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45(3):321–325. - PubMed
-
- Khoo CP, Pozzilli P, Alison MR. Endothelial progenitor cells and their potential therapeutic applications. Regenerative Medicine. 2008;3(6):863–876. - PubMed
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. - PubMed
-
- Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197(2):496–503. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
