Protection against SHIV-KB9 infection by combining rDNA and rFPV vaccines based on HIV multiepitope and p24 protein in Chinese rhesus macaques
- PMID: 22474488
- PMCID: PMC3299295
- DOI: 10.1155/2012/958404
Protection against SHIV-KB9 infection by combining rDNA and rFPV vaccines based on HIV multiepitope and p24 protein in Chinese rhesus macaques
Abstract
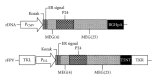
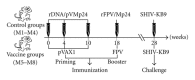
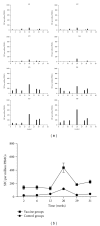
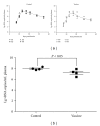
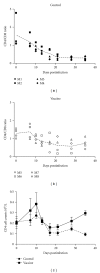
Developing an effective vaccine against HIV infection remains an urgent goal. We used a DNA prime/fowlpox virus boost regimen to immunize Chinese rhesus macaques. The animals were challenged intramuscularly with pathogenic molecularly cloned SHIV-KB9. Immunogenicity and protective efficacy of vaccines were investigated by measuring IFN-γ levels, monitoring HIV-specific binding antibodies, examining viral load, and analyzing CD4/CD8 ratio. Results show that, upon challenge, the vaccine group can induce a strong immune response in the body, represented by increased expression of IFN-γ, slow and steady elevated antibody production, reduced peak value of acute viral load, and increase in the average CD4/CD8 ratio. The current research suggests that rapid reaction speed, appropriate response strength, and long-lasting immune response time may be key protection factors for AIDS vaccine. The present study contributes significantly to AIDS vaccine and preclinical research.
Figures
Similar articles
-
Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIVSF162P3.Vaccine. 2005 Oct 10;23(42):5009-21. doi: 10.1016/j.vaccine.2005.05.032. Vaccine. 2005. PMID: 15985317
-
Efficacy of DNA and fowlpox virus priming/boosting vaccines for simian/human immunodeficiency virus.J Virol. 2004 Dec;78(24):13819-28. doi: 10.1128/JVI.78.24.13819-13828.2004. J Virol. 2004. PMID: 15564490 Free PMC article.
-
Protective effects of nef-deleted SHIV or that having IFN-gamma against disease induced with a pathogenic virus early after vaccination.Arch Virol. 2004 Sep;149(9):1705-20. doi: 10.1007/s00705-004-0333-8. Arch Virol. 2004. PMID: 15593414
-
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models.Expert Rev Vaccines. 2017 Oct;16(10):973-985. doi: 10.1080/14760584.2017.1371594. Epub 2017 Sep 4. Expert Rev Vaccines. 2017. PMID: 28838267 Free PMC article. Review.
-
Development of a DNA-MVA/HIVA vaccine for Kenya.Vaccine. 2002 May 6;20(15):1995-8. doi: 10.1016/s0264-410x(02)00085-3. Vaccine. 2002. PMID: 11983261 Review.
Cited by
-
Overcoming immunogenicity issues of HIV p24 antigen by the use of innovative nanostructured lipid carriers as delivery systems: evidences in mice and non-human primates.NPJ Vaccines. 2018 Oct 1;3:46. doi: 10.1038/s41541-018-0086-0. eCollection 2018. NPJ Vaccines. 2018. PMID: 30302284 Free PMC article.
-
A phase 1, randomized, controlled dose-escalation study of EP-1300 polyepitope DNA vaccine against Plasmodium falciparum malaria administered via electroporation.Vaccine. 2016 Nov 4;34(46):5571-5578. doi: 10.1016/j.vaccine.2016.09.041. Epub 2016 Sep 30. Vaccine. 2016. PMID: 27697302 Free PMC article. Clinical Trial.
-
Generation and evaluation of clade C simian-human immunodeficiency virus challenge stocks.J Virol. 2015 Feb;89(4):1965-74. doi: 10.1128/JVI.03279-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473043 Free PMC article.
-
In silico design of a DNA-based HIV-1 multi-epitope vaccine for Chinese populations.Hum Vaccin Immunother. 2015;11(3):795-805. doi: 10.1080/21645515.2015.1012017. Hum Vaccin Immunother. 2015. PMID: 25839222 Free PMC article.
-
The evolution of poxvirus vaccines.Viruses. 2015 Apr 7;7(4):1726-803. doi: 10.3390/v7041726. Viruses. 2015. PMID: 25853483 Free PMC article. Review.
References
-
- Sinoussi FB, Chermann JC, Rey F. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. - PubMed
-
- Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24(19):4062–4081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
