Transient Silencing of a Type IV P-Type ATPase, Atp10c, Results in Decreased Glucose Uptake in C2C12 Myotubes
- PMID: 22474575
- PMCID: PMC3317196
- DOI: 10.1155/2012/152902
Transient Silencing of a Type IV P-Type ATPase, Atp10c, Results in Decreased Glucose Uptake in C2C12 Myotubes
Abstract
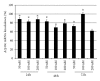
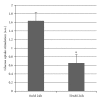
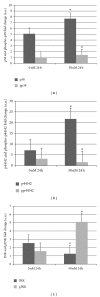
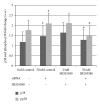
Atp10c is a strong candidate gene for diet-induced obesity and type 2 diabetes. To identify molecular and cellular targets of ATP10C, Atp10c expression was altered in vitro in C2C12 skeletal muscle myotubes by transient transfection with an Atp10c-specific siRNA. Glucose uptake assays revealed that insulin stimulation caused a significant 2.54-fold decrease in 2-deoxyglucose uptake in transfected cells coupled with a significant upregulation of native mitogen-activated protein kinases (MAPKs), p38, and p44/42. Additionally, glucose transporter-1 (GLUT1) was significantly upregulated; no changes in glucose transporter-4 (GLUT4) expression were observed. The involvement of MAPKs was confirmed using the specific inhibitor SB203580, which downregulated the expression of native and phosphorylated MAPK proteins in transfected cells without any changes in insulin-stimulated glucose uptake. Results indicate that Atp10c regulates glucose metabolism, at least in part via the MAPK pathway, and, thus, plays a significant role in the development of insulin resistance and type 2 diabetes.
Figures


Similar articles
-
A type IV P-type ATPase affects insulin-mediated glucose uptake in adipose tissue and skeletal muscle in mice.J Nutr Biochem. 2006 Dec;17(12):811-20. doi: 10.1016/j.jnutbio.2006.01.002. Epub 2006 Feb 3. J Nutr Biochem. 2006. PMID: 16517145
-
GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase.Biochem J. 2001 Nov 1;359(Pt 3):639-49. doi: 10.1042/0264-6021:3590639. Biochem J. 2001. PMID: 11672439 Free PMC article.
-
The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation.Diabetes. 2001 Jun;50(6):1464-71. doi: 10.2337/diabetes.50.6.1464. Diabetes. 2001. PMID: 11375349
-
Alisol A-24-acetate promotes glucose uptake via activation of AMPK in C2C12 myotubes.BMC Complement Med Ther. 2020 Jan 29;20(1):22. doi: 10.1186/s12906-019-2802-3. BMC Complement Med Ther. 2020. PMID: 32020870 Free PMC article.
-
Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis.J Biol Chem. 2003 May 16;278(20):17953-62. doi: 10.1074/jbc.M211136200. Epub 2003 Mar 11. J Biol Chem. 2003. PMID: 12637564
Cited by
-
Deficiency of the lipid flippase ATP10A causes diet-induced dyslipidemia in female mice.Sci Rep. 2024 Jan 3;14(1):343. doi: 10.1038/s41598-023-50360-5. Sci Rep. 2024. PMID: 38172157 Free PMC article.
-
Subcongenic analysis of a quantitative trait locus affecting body weight and glucose metabolism in zinc transporter 7 (znt7)-knockout mice.BMC Genet. 2019 Feb 18;20(1):19. doi: 10.1186/s12863-019-0715-2. BMC Genet. 2019. PMID: 30777014 Free PMC article.
-
Substrates, regulation, cellular functions, and disease associations of P4-ATPases.Commun Biol. 2025 Jan 28;8(1):135. doi: 10.1038/s42003-025-07549-3. Commun Biol. 2025. PMID: 39875509 Free PMC article. Review.
-
P4 ATPases: flippases in health and disease.Int J Mol Sci. 2013 Apr 11;14(4):7897-922. doi: 10.3390/ijms14047897. Int J Mol Sci. 2013. PMID: 23579954 Free PMC article. Review.
-
Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport.Biochim Biophys Acta. 2013 Mar;1831(3):555-74. doi: 10.1016/j.bbalip.2012.10.006. Epub 2012 Oct 26. Biochim Biophys Acta. 2013. PMID: 23103747 Free PMC article. Review.
References
-
- Hayata K, Sakano K, Nishinaka S. Establishment of new highly insulin-sensitive cell lines and screening of compounds to facilitate glucose consumption. Journal of Pharmacological Sciences. 2008;108(3):348–354. - PubMed
-
- Dhar MS, Sommardahl CS, Kirkland T, et al. Mice heterozygous for atp10c, a putative amphipath, represent a novel model of obesity and type 2 diabetes. The Journal of Nutrition. 2004;134(4):799–805. - PubMed
-
- Dhar M, Webb LS, Smith L, Hauser L, Johnson D, West DB. A novel ATPase on mouse chromosome 7 is a candidate gene for increased body fat. Physiol Genomics. 2000;4(1):93–100. - PubMed
-
- Meguro M, Kashiwagi A, Mitsuya K, et al. A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nature Genetics. 2001;28(1):19–20. - PubMed
-
- Dhar MS, Yuan JS, Elliott SB, Sommardahl C. A type IV P-type ATPase affects insulin-mediated glucose uptake in adipose tissue and skeletal muscle in mice. Journal of Nutritional Biochemistry. 2006;17(12):811–820. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

