Brain imaging in Alzheimer disease
- PMID: 22474610
- PMCID: PMC3312396
- DOI: 10.1101/cshperspect.a006213
Brain imaging in Alzheimer disease
Abstract
Imaging has played a variety of roles in the study of Alzheimer disease (AD) over the past four decades. Initially, computed tomography (CT) and then magnetic resonance imaging (MRI) were used diagnostically to rule out other causes of dementia. More recently, a variety of imaging modalities including structural and functional MRI and positron emission tomography (PET) studies of cerebral metabolism with fluoro-deoxy-d-glucose (FDG) and amyloid tracers such as Pittsburgh Compound-B (PiB) have shown characteristic changes in the brains of patients with AD, and in prodromal and even presymptomatic states that can help rule-in the AD pathophysiological process. No one imaging modality can serve all purposes as each have unique strengths and weaknesses. These modalities and their particular utilities are discussed in this article. The challenge for the future will be to combine imaging biomarkers to most efficiently facilitate diagnosis, disease staging, and, most importantly, development of effective disease-modifying therapies.
Figures
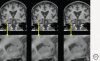
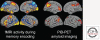
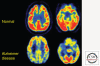
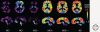
References
-
- Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA 1996. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 47: 462–466 - PubMed
-
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB 1997. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: Implications for the cognitive reserve hypothesis. Am J Psychiatry 154: 165–172 - PubMed
-
- Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, Nordberg A 2001. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport 12: 851–855 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
