Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease
- PMID: 22475209
- PMCID: PMC3348060
- DOI: 10.1186/1750-1326-7-12
Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington's disease
Abstract
Background: No disease modifying treatment currently exists for Huntington's disease (HD), a fatal neurodegenerative disorder characterized by the formation of amyloid-like aggregates of the mutated huntingtin protein. Curcumin is a naturally occurring polyphenolic compound with Congo red-like amyloid binding properties and the ability to cross the blood brain barrier. CAG140 mice, a knock-in (KI) mouse model of HD, display abnormal aggregates of mutant huntingtin and striatal transcriptional deficits, as well as early motor, cognitive and affective abnormalities, many months prior to exhibiting spontaneous gait deficits, decreased striatal volume, and neuronal loss. We have examined the ability of life-long dietary curcumin to improve the early pathological phenotype of CAG140 mice.
Results: KI mice fed a curcumin-containing diet since conception showed decreased huntingtin aggregates and increased striatal DARPP-32 and D1 receptor mRNAs, as well as an amelioration of rearing deficits. However, similar to other antioxidants, curcumin impaired rotarod behavior in both WT and KI mice and climbing in WT mice. These behavioral effects were also noted in WT C57Bl/6 J mice exposed to the same curcumin regime as adults. However, neither locomotor function, behavioral despair, muscle strength or food utilization were affected by curcumin in this latter study. The clinical significance of curcumin's impairment of motor performance in mice remains unclear because curcumin has an excellent blood chemistry and adverse event safety profile, even in the elderly and in patients with Alzheimer's disease.
Conclusion: Together with this clinical experience, the improvement in several transgene-dependent parameters by curcumin in our study supports a net beneficial effect of dietary curcumin in HD.
Figures

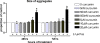





Similar articles
-
Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington's disease.Mol Cell Neurosci. 2012 Feb;49(2):149-57. doi: 10.1016/j.mcn.2011.10.007. Epub 2011 Oct 20. Mol Cell Neurosci. 2012. PMID: 22044764 Free PMC article.
-
Sex differences in behavior and striatal ascorbate release in the 140 CAG knock-in mouse model of Huntington's disease.Behav Brain Res. 2007 Mar 12;178(1):90-7. doi: 10.1016/j.bbr.2006.12.004. Epub 2007 Jan 18. Behav Brain Res. 2007. PMID: 17239451 Free PMC article.
-
Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington's disease.Hum Mol Genet. 2019 Dec 1;28(23):4012-4021. doi: 10.1093/hmg/ddz247. Hum Mol Genet. 2019. PMID: 31630202
-
Longitudinal behavioral, cross-sectional transcriptional and histopathological characterization of a knock-in mouse model of Huntington's disease with 140 CAG repeats.Exp Neurol. 2011 Apr;228(2):173-82. doi: 10.1016/j.expneurol.2010.12.017. Epub 2010 Dec 28. Exp Neurol. 2011. PMID: 21192926 Free PMC article.
-
Treadmill exercise delays the onset of non-motor behaviors and striatal pathology in the CAG140 knock-in mouse model of Huntington's disease.Neurobiol Dis. 2017 Sep;105:15-32. doi: 10.1016/j.nbd.2017.05.004. Epub 2017 May 11. Neurobiol Dis. 2017. PMID: 28502806
Cited by
-
Curcumin modulates α-synuclein aggregation and toxicity.ACS Chem Neurosci. 2013 Mar 20;4(3):393-407. doi: 10.1021/cn3001203. Epub 2012 Dec 17. ACS Chem Neurosci. 2013. PMID: 23509976 Free PMC article.
-
Reactive Species in Huntington Disease: Are They Really the Radicals You Want to Catch?Antioxidants (Basel). 2020 Jul 2;9(7):577. doi: 10.3390/antiox9070577. Antioxidants (Basel). 2020. PMID: 32630706 Free PMC article. Review.
-
Curcumin, inflammation, and neurological disorders: How are they linked?Integr Med Res. 2023 Sep;12(3):100968. doi: 10.1016/j.imr.2023.100968. Epub 2023 Jun 25. Integr Med Res. 2023. PMID: 37664456 Free PMC article. Review.
-
Can Curcumin Counteract Cognitive Decline? Clinical Trial Evidence and Rationale for Combining ω-3 Fatty Acids with Curcumin.Adv Nutr. 2018 Mar 1;9(2):105-113. doi: 10.1093/advances/nmx013. Adv Nutr. 2018. PMID: 29659685 Free PMC article. Review.
-
Bibliometric Analysis of Curcumin Based on CiteSpace: Landscapes, Hotspots, and Frontiers.Drug Des Devel Ther. 2024 Dec 6;18:5743-5758. doi: 10.2147/DDDT.S494758. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39659947 Free PMC article. Review.
References
-
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ. et al.Detection of Huntington's disease decades before diagnosis: The Predict HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. - DOI - PMC - PubMed
-
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI. et al.Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

