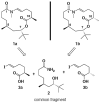
Asymmetric total synthesis and absolute stereochemistry of the neuroactive marine macrolide palmyrolide A
- PMID: 22475318
- PMCID: PMC3352666
- DOI: 10.1021/ol300673m
Asymmetric total synthesis and absolute stereochemistry of the neuroactive marine macrolide palmyrolide A
Abstract
The first asymmetric total synthesis and determination of the absolute configuration for the neuroactive marine macrolide palmyrolide A is described. The highlight of the synthesis is macrocyclization via trans-enamide formation catalyzed by copper(I) iodide and cesium carbonate. Comparison with the authentic spectral data confirms the synthesis of (+)-ent-palmyrolide A.
© 2012 American Chemical Society
Figures
References
-
-
Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. J Org Chem. 1999;64:866–876.. (b) This method was successfully applied to the structure elucidation of the (−)-apratoxin A macrolide. See reference .
-
-
- Luesch H, Yoshida WY, Moore RE, Paul VJ, Corbett TH. J Am Chem Soc. 2001;123:5418–5423. - PubMed
- Klein D, Braekman JC, Daloze D, Hoffmann L, Castillo G, Demoulin V. J Nat Prod. 1999;62:934–936. - PubMed
- Matthew S, Salvador LA, Schupp PJ, Paul VJ, Luesch H. J Nat Prod. 2010;73:1544–1552. - PMC - PubMed
-
-
For the most recent total synthesis, see: Numajiri Y, Takahashi T, Doi T. Chem Asian J. 2009;4:111–125.. For the first total synthesis, see: Chen J, Forsyth CJ. J Am Chem Soc. 2003;125:8734–8735.
-
-
-
For the apratoxin numbering convention, see reference .
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous