Plastid division
- PMID: 22476074
- PMCID: PMC2995336
- DOI: 10.1093/aobpla/plq016
Plastid division
Abstract
Background and aims: Plastids undergo a process of binary fission in order to replicate. Plastid replication is required at two distinct stages of plant growth: during cell division to ensure correct plastid segregation, and during cell expansion and development to generate large populations of functional plastids, as in leaf mesophyll cells. This review considers some of the recent advances in the understanding of how plastids undergo binary fission, a process which uses several different proteins, both internal and external to the plastid, which have been derived from the original endosymbiont's genome as well as new proteins that have been recruited from the host genome.
Key points: Several of the proteins currently used in this process in higher plants have homologues in modern-day bacteria. An alternative mode of replication by a budding-type mechanism also appears to be used in some circumstances. The review also highlights how most of our knowledge of plastid division is centred on the chloroplast developing in leaf mesophyll cells and a role for plastid division during the development of other plastid types is poorly understood. Whilst models for a protein-based mechanism have been devised, exactly how the division process is controlled at the plastid level and at the plastid population level is poorly understood.
Figures
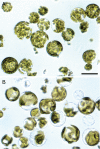
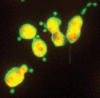
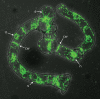
References
-
- Bechtel DB, Wilson JD. Amyloplast formation and starch granule development in hard red winter wheat. Cereal Chemistry. 2003;80:175–183. doi:10.1094/CCHEM.2003.80.2.175. - DOI
-
- Chaley N, Possingham JV. Structure of constricted proplastids in meristematic plant tissues. Biologie Cellulaire. 1981;41:203–210.
-
- Chen Y, Asano T, Fujiwara MT, Yoshida S, Machida Y, Yoshiok Y. Plant cells without detectable plastids are generated in the crumpled leaf mutant of Arabidopsis thaliana. Plant and Cell Physiology. 2009;50:956–969. doi:10.1093/pcp/pcp047. - DOI - PubMed
-
- Colletti KS, Tattersall EA, Pyke KA, Froelich JE, Stokes KD, Osteryoung KW. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Current Biology. 2000;10:507–516. doi:10.1016/S0960-9822(00)00466-8. - DOI - PubMed
-
- Cookson PJ, Kiano JW, Shipton CA, Fraser PD, Romer S, Schuch W, Bramley PM, Pyke KA. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta. 2003;217:896–903. doi:10.1007/s00425-003-1065-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

