Methods and recommendations for evaluating and reporting a new diagnostic test
- PMID: 22476385
- PMCID: PMC3661219
- DOI: 10.1007/s10096-012-1602-1
Methods and recommendations for evaluating and reporting a new diagnostic test
Abstract
No standardized guidelines exist for the biostatistical methods appropriate for studies evaluating diagnostic tests. Publication recommendations such as the STARD statement provide guidance for the analysis of data, but biostatistical advice is minimal and application is inconsistent. This article aims to provide a self-contained, accessible resource on the biostatistical aspects of study design and reporting for investigators. For all dichotomous diagnostic tests, estimates of sensitivity and specificity should be reported with confidence intervals. Power calculations are strongly recommended to ensure that investigators achieve desired levels of precision. In the absence of a gold standard reference test, the composite reference standard method is recommended for improving estimates of the sensitivity and specificity of the test under evaluation.
Conflict of interest statement
Figures
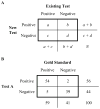

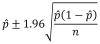
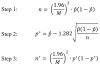


References
-
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Intern Med. 2003;138(1):40–44. - PubMed
-
- Pfeifer J, editor. Molecular genetic testing in surgical pathology. Lippincott Williams & Wilkins; Philadelphia: 2006.
-
- Rosner BA. Fundamentals of biostatistics. 6. Thomson Brooks Cole; Belmont, CA: 2006.
-
- FDA. Statistical guidance on reporting results from studies evaluating diagnostic tests. 2011 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDo.... Updated 6 January 2011; cited 8 December 2011.
-
- Royse D, Thyer BA, Padgett DK. Program evaluation: An introduction. 5. Wadsworth, Cengage Learning; Belmont, CA: 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

