Potential role of high molecular weight calmodulin-binding protein in cardiac injury
- PMID: 22477545
- PMCID: PMC2903026
- DOI: 10.1055/s-0031-1278346
Potential role of high molecular weight calmodulin-binding protein in cardiac injury
Abstract
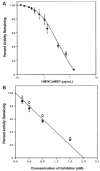
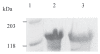
Ca(2+) is a major determinant of many biochemical processes in various cell types and is a critical second messenger in cell signalling. High molecular weight calmodulin-binding protein (HMWCaMBP) was originally discovered and purified in the authors' laboratory. It was identified as a homologue of calpastatin - an inhibitor of Ca(2+)-activated cysteine proteases (calpains). Decreased expression of HMWCaMBP in ischemia suggests that it is proteolyzed by calpains during ischemia and reperfusion. In normal myocardial muscle, HMWCaMBP may protect its substrate from calpains, but during an early stage of ischemia/reperfusion with increased Ca(2+) influx, calpain activity exceeds HMWCaMBP activity, leading to proteolysis of HMWCaMBP and other protein substrates, resulting in cellular damage. The role of HMWCaMBP in ischemia/reperfusion is yet to be explored. The present review summarizes developments from the authors' laboratory in the area of HMWCaMBP.
Figures





Similar articles
-
High molecular weight calmodulin-binding protein: 20 years onwards-a potential therapeutic calpain inhibitor.Cardiovasc Drugs Ther. 2012 Aug;26(4):321-30. doi: 10.1007/s10557-012-6399-8. Cardiovasc Drugs Ther. 2012. PMID: 22588788 Review.
-
Decreased expression of high-molecular-weight calmodulin-binding protein and its correlation with apoptosis in ischemia-reperfused rat heart.Cell Calcium. 2001 Jan;29(1):59-71. doi: 10.1054/ceca.2000.0157. Cell Calcium. 2001. PMID: 11133356
-
Altered expression of high-molecular-weight calmodulin-binding protein in human ischaemic myocardium.J Pathol. 2000 Jun;191(2):208-16. doi: 10.1002/(SICI)1096-9896(200006)191:2<208::AID-PATH618>3.0.CO;2-6. J Pathol. 2000. PMID: 10861583
-
Ischemia and reperfusion induce differential expression of calpastatin and its homologue high molecular weight calmodulin-binding protein in murine cardiomyocytes.PLoS One. 2014 Dec 8;9(12):e114653. doi: 10.1371/journal.pone.0114653. eCollection 2014. PLoS One. 2014. PMID: 25486053 Free PMC article.
-
Calmodulin-binding proteins: A journey of 40 years.Cell Calcium. 2018 Nov;75:89-100. doi: 10.1016/j.ceca.2018.09.002. Epub 2018 Sep 5. Cell Calcium. 2018. PMID: 30205293 Review.
Cited by
-
Expression of calcineurin, calpastatin and heat shock proteins during ischemia and reperfusion.Biochem Biophys Rep. 2015 Sep 25;4:207-214. doi: 10.1016/j.bbrep.2015.09.016. eCollection 2015 Dec. Biochem Biophys Rep. 2015. PMID: 30338302 Free PMC article.
References
-
- Cheung WY. Calmodulin plays a pivotal role in cellular regulation. Science. 1980;207:19–27. - PubMed
-
- Klee CB. Interaction of CaM with calcium and target proteins. In: Cohen P, Klee CB, editors. Molecular Aspects of Cellular Regulation. Vol. 5. Amsterdam: Elsevier; 1988. pp. 35–56.
-
- Rogers MS, Strehler EE. Calmodulin. In: Celio MR, Pauls T, Schwaller B, editors. Guidebook to the Calcium Binding Proteins. Oxford: Sambrook and Tooze, Oxford University Press; 1996. pp. 34–40.
-
- Yokoyama N, Wang JH. Demonstration and purification of multiple bovine brain and bovine lung calmodulin-stimulated phosphatase isozymes. J Biol Chem. 1991;266:14822–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

