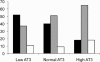Effects of age and weight-based dosing of enoxaparin on anti-factor xa levels in pediatric patients
- PMID: 22477802
- PMCID: PMC3018183
Effects of age and weight-based dosing of enoxaparin on anti-factor xa levels in pediatric patients
Abstract
Objective: The objective of this dose range study is to expand on the relationship between age and weight-based doses of enoxaparin and resulting levels of anti-factor Xa (anti-Xa) in pediatric patients. The primary outcome of this study is to determine the average dose of enoxaparin required to produce a therapeutic effect. Secondary outcomes include the number of enoxaparin dose changes required to achieve a therapeutic level of anti-Xa in each age group, the success rates of achieving and maintaining therapeutic anti-Xa levels, and the effect of serum antithrombin concentrations on anti-Xa levels. The study will also determine whether different dispensed concentrations of enoxaparin play a role in achieving therapeutic levels of anti-Xa.
Methods: Single center, retrospective chart review. Patients were excluded from the study if they were older than 18 years of age, were receiving enoxaparin for prophylactic purposes, had a creatinine clearance < 30 ml/min/1.73m(2), and if no anti-factor Xa levels were drawn.
Results: Average enoxaparin doses required for therapeutic levels of anti-factor Xa were 1.8 mg/kg for patients <1 month, 1.64 mg/kg (1 month to 1 year), 1.45 mg/kg (1 to 6 years), and 1.05 mg/kg (>6 years of age). An average of 3.24 dose changes was required for neonates to achieve therapeutic levels anti-factor Xa. The success rates for achieving and maintaining therapeutic levels were both 41%. Patients with low serum antithrombin levels were more likely to have low anti-Xa levels than those with normal or high values, 52% vs 40% vs 18%, respectively. Patients receiving diluted concentrations, 10 or 20 mg/mL, experienced lower anti-Xa levels than patients who received the standard manufactured concentration of 100 mg/mL, 61% vs 33%.
Conclusion: Based on this dose-range study, enoxaparin should be initiated at larger doses than recommended by the current guidelines to promptly achieve therapeutic anti-Xa levels. Doses should be divided into three age groups instead of two as currently suggested in the guidelines. To increase the likelihood of achieving therapeutic levels, the commercially available enoxaparin product should not be diluted if possible.
Figures
Similar articles
-
An Evaluation of the Appropriateness of Initial Enoxaparin Dosing Among Pediatric Patients.Cureus. 2023 Nov 15;15(11):e48830. doi: 10.7759/cureus.48830. eCollection 2023 Nov. Cureus. 2023. PMID: 38106767 Free PMC article.
-
Higher doses of low-molecular-weight heparin (enoxaparin) are needed to achieve target anti-Xa concentrations in critically ill children*.Pediatr Crit Care Med. 2014 Sep;15(7):e294-9. doi: 10.1097/PCC.0000000000000169. Pediatr Crit Care Med. 2014. PMID: 24901803
-
Retrospective evaluation of enoxaparin dosing in patients 48 weeks' postmenstrual age or younger in a neonatal intensive care unit.Ann Pharmacother. 2012 Jul-Aug;46(7-8):943-51. doi: 10.1345/aph.1R116. Epub 2012 Jul 24. Ann Pharmacother. 2012. PMID: 22828970
-
Utility of anti-factor Xa monitoring in surgical patients receiving prophylactic doses of enoxaparin for venous thromboembolism prophylaxis.Am J Surg. 2017 Jun;213(6):1143-1152. doi: 10.1016/j.amjsurg.2016.08.010. Epub 2016 Sep 2. Am J Surg. 2017. PMID: 27692434 Review.
-
Review of current evidence available for guiding optimal Enoxaparin prophylactic dosing strategies in obese patients-Actual Weight-based vs Fixed.Crit Rev Oncol Hematol. 2017 May;113:191-194. doi: 10.1016/j.critrevonc.2017.03.022. Epub 2017 Mar 22. Crit Rev Oncol Hematol. 2017. PMID: 28427508 Review.
Cited by
-
An Evaluation of the Appropriateness of Initial Enoxaparin Dosing Among Pediatric Patients.Cureus. 2023 Nov 15;15(11):e48830. doi: 10.7759/cureus.48830. eCollection 2023 Nov. Cureus. 2023. PMID: 38106767 Free PMC article.
-
Balancing safety and efficacy of low-molecular-weight heparins in neonates: a systematic review.Res Pract Thromb Haemost. 2024 Oct 23;8(7):102601. doi: 10.1016/j.rpth.2024.102601. eCollection 2024 Oct. Res Pract Thromb Haemost. 2024. PMID: 39619814 Free PMC article. Review.
-
Evaluation of Initial Enoxaparin Dosing and Antifactor Xa Levels in Infants Admitted to the Neonatal Intensive Care Unit.Biomed Hub. 2024 Apr 12;9(1):54-61. doi: 10.1159/000537797. eCollection 2024 Jan-Dec. Biomed Hub. 2024. PMID: 38616894 Free PMC article.
-
American Society of Hematology/International Society on Thrombosis and Haemostasis 2024 updated guidelines for treatment of venous thromboembolism in pediatric patients.Blood Adv. 2025 May 27;9(10):2587-2636. doi: 10.1182/bloodadvances.2024015328. Blood Adv. 2025. PMID: 40423983 Free PMC article.
-
Admission thrombelastography does not guide dose adjustment of enoxaparin in trauma patients.Surg Open Sci. 2020 Apr 14;2(4):41-44. doi: 10.1016/j.sopen.2020.03.003. eCollection 2020 Oct. Surg Open Sci. 2020. PMID: 33073224 Free PMC article.
References
-
- Punzalan RC, Hillery CA, Montgomery RR, et al. Low molecular weight heparin in thrombotic disease in children and adolescents. J Pediatr Hematol Oncol. 2000;22:137–142. - PubMed
-
- Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for thromboembolic disease. Chest. 2001;119:176S–193S. - PubMed
-
- Fareed J, Hoppensteadt D, Walenga J, et al. Pharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practice. Clin Pharmacokinet. 2003;42:1043–57. - PubMed
-
- Malowany JI, Knoppert DC, Chan AKC, et al. Enoxaparin use in the neonatal intensive care unit: experience over 8 years. Pharmacotherapy. 2007;27:1263–1271. - PubMed
-
- Monagle P, Michelson AD, Bovill E, et al. Antithrombotic therapy in children. Chest. 2001;119:344–370. - PubMed
LinkOut - more resources
Full Text Sources