Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies
- PMID: 22479384
- PMCID: PMC3314002
- DOI: 10.1371/journal.pone.0033328
Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies
Abstract
Background: Accurate and reliable laboratory methods are needed for estimation of HIV-1 incidence to identify the high-risk populations and target and monitor prevention efforts. We previously described a single-well limiting-antigen avidity enzyme immunoassay (LAg-Avidity EIA) to detect recent HIV-1 infection.
Methods: We describe here further optimization and characterization of LAg-Avidity EIA, comparing it to the BED assay and a two-well avidity-index (AI) EIA. Specimen sets included longitudinal sera (n = 393), collected from 89 seroconverting individuals from 4 cohorts representing 4 HIV-1 subtypes, and sera from AIDS patients (n = 488) with or without TB co-infections from 3 different cohorts. Ninety seven HIV-1 positive specimens were purchased commercially. The BED assay, LAg-Avidity EIA, AI-EIA and HIV serology were performed, as needed.
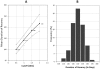
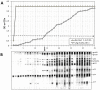
Results: Monitoring quality control specimens indicated high reproducibility of the LAg-Avidity EIA with coefficient of variation of <10% in the dynamic range. The LAg-Avidity EIA has an overall mean duration of recency (ω) of 141 days (95% CI 119-160) at normalized optical density (ODn) cutoff of 1.0, with similar ω in different HIV-1 subtypes and populations (132 to 143 days). Antibody avidity kinetics were similar among individuals and subtypes by both the LAg-Avidity EIA and AI-EIA compared to the HIV-IgG levels measured by the BED assay. The false recent rate among individuals with AIDS was 0.2% with the LAg-Avidity EIA, compared to 2.9% with the BED assay. Western blot profiles of specimens with increasing avidity confirm accurate detection of recent HIV-1 infections.
Conclusions: These data demonstrate that the LAg-Avidity EIA is a promising assay with consistent ω in different populations and subtypes. The assay should be very useful for 1) estimating HIV-1 incidence in cross-sectional specimens as part of HIV surveillance, 2) identifying risk factors for recent infections, 3) measuring impact of prevention programs, and 4) studying avidity maturation during vaccine trials.
Conflict of interest statement
Figures


References
-
- Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010;24:2763–2771. - PubMed
-
- Janssen RS, Satten GA, Stramer SL, Rawal BD, O'Brien TR, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. [erratum appears in JAMA 1999 May 26;281(20):1893]. JAMA. 1998;280:42–48. - PubMed
-
- Parekh BS, Pau CP, Kennedy MS, Dobbs TL, McDougal JS. Assessment of antibody assays for identifying and distinguishing recent from long-term HIV type 1 infection. AIDS Research & Human Retroviruses. 2001;17:137–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

