Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs
- PMID: 22479411
- PMCID: PMC3316581
- DOI: 10.1371/journal.pone.0033559
Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs
Abstract
Background: We aimed to determine the representation of elderly people in published reports of randomized controlled trials (RCTs). We focused on trials of 4 medications--pioglitazone, rosuvastatin, risedronate, and valsartan-frequently used by elderly patients with chronic medical conditions.
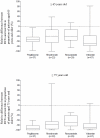
Methods and findings: We selected all reports of RCTs indexed in PubMed from 1966 to April 2008 evaluating one of the 4 medications of interest. Estimates of the community-based "on-treatment" population were from a national health insurance database (SNIIR-AM) covering approximately 86% of the population in France. From this database, we evaluated data claims from January 2006 to December 2007 for 1,958,716 patients who received one of the medications of interest for more than 6 months. Of the 155 RCT reports selected, only 3 studies were exclusively of elderly patients (2 assessing valsartan; 1 risedronate). In only 4 of 37 reports (10.8%) for pioglitazone, 4 of 22 (18.2%) for risedronate, 3 of 29 (10.3%) for rosuvastatine and 9 of 67 (13.4%) for valsartan, the proportion of patients aged 65 or older was within or above that treated in clinical practice. In 62.2% of the reports for pioglitazone, 40.9% for risedronate, 37.9% for rosuvastatine, and 70.2% for valsartan, the proportion of patients aged 65 or older was lower than half that in the treated population. The representation of elderly people did not differ by publication date or sample size.
Conclusions: Elderly patients are poorly represented in RCTs of drugs they are likely to receive.
Conflict of interest statement
Figures


References
-
- Commitee on Comparative Research Prioritization. Institute of Medicine Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academy Press; 2009.
-
- United Nations. World Population to 2300. 2004. Department of Economic and Social Affairs, Population Division New York.
-
- European Commission. 2009 Ageing report. European Commision website. 2009. Available: http://ec.europa.eu/economy_finance/publications/publication14992_en.pdf. Accessed 2012 Feb 27.
-
- Fahy N, McKee M, Busse R, Grundy E. How to meet the challenge of ageing populations. Bmj. 2011;342:d3815. - PubMed
-
- Jylha M. Ten-year change in the use of medical drugs among the elderly–a longitudinal study and cohort comparison. J Clin Epidemiol. 1994;47:69–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

