Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts
- PMID: 22479469
- PMCID: PMC3315513
- DOI: 10.1371/journal.pone.0033894
Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts
Abstract
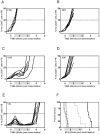
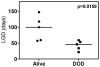
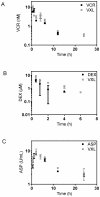
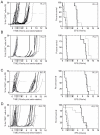
Current regimens for induction therapy of pediatric acute lymphoblastic leukemia (ALL), or for re-induction post relapse, use a combination of vincristine (VCR), a glucocorticoid, and L-asparaginase (ASP) with or without an anthracycline. With cure rates now approximately 80%, robust pre-clinical models are necessary to prioritize active new drugs for clinical trials in relapsed/refractory patients, and the ability of these models to predict synergy/antagonism with established therapy is an essential attribute. In this study, we report optimization of an induction-type regimen by combining VCR, dexamethasone (DEX) and ASP (VXL) against ALL xenograft models established from patient biopsies in immune-deficient mice. We demonstrate that the VXL combination was synergistic in vitro against leukemia cell lines as well as in vivo against ALL xenografts. In vivo, VXL treatment caused delays in progression of individual xenografts ranging from 22 to >146 days. The median progression delay of xenografts derived from long-term surviving patients was 2-fold greater than that of xenografts derived from patients who died of their disease. Pharmacokinetic analysis revealed that systemic DEX exposure in mice increased 2-fold when administered in combination with VCR and ASP, consistent with clinical findings, which may contribute to the observed synergy between the 3 drugs. Finally, as proof-of-principle we tested the in vivo efficacy of combining VXL with either the Bcl-2/Bcl-xL/Bcl-w inhibitor, ABT-737, or arsenic trioxide to provide evidence of a robust in vivo platform to prioritize new drugs for clinical trials in children with relapsed/refractory ALL.
Conflict of interest statement
Figures



References
-
- Pui CH, Pui C-H. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010;109:777–787. - PubMed
-
- Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. - PubMed
-
- Chessells JM, Veys P, Kempski H, Henley P, Leiper A, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

