Xylosyltransferase-I regulates glycosaminoglycan synthesis during the pathogenic process of human osteoarthritis
- PMID: 22479506
- PMCID: PMC3316535
- DOI: 10.1371/journal.pone.0034020
Xylosyltransferase-I regulates glycosaminoglycan synthesis during the pathogenic process of human osteoarthritis
Abstract
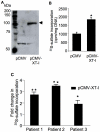
Loss of glycosaminoglycan (GAG) chains of proteoglycans (PGs) is an early event of osteoarthritis (OA) resulting in cartilage degradation that has been previously demonstrated in both huma and experimental OA models. However, the mechanism of GAG loss and the role of xylosyltransferase-I (XT-I) that initiates GAG biosynthesis onto PG molecules in the pathogenic process of human OA are unknown. In this study, we have characterized XT-I expression and activity together with GAG synthesis in human OA cartilage obtained from different regions of the same joint, defined as "normal", "late-stage" or adjacent to "late-stage". The results showed that GAG synthesis and content increased in cartilage from areas flanking OA lesions compared to cartilage from macroscopically "normal" unaffected regions, while decreased in "late-stage" OA cartilage lesions. This increase in anabolic state was associated with a marked upregulation of XT-I expression and activity in cartilage "next to lesion" while a decrease in the "late-stage" OA cartilage. Importantly, XT-I inhibition by shRNA or forced-expression with a pCMV-XT-I construct correlated with the modulation of GAG anabolism in human cartilage explants. The observation that XT-I gene expression was down-regulated by IL-1β and up-regulated by TGF-β1 indicates that these cytokines may play a role in regulating GAG content in human OA. Noteworthy, expression of IL-1β receptor (IL-1R1) was down-regulated whereas that of TGF-β1 was up-regulated in early OA cartilage. Theses observations may account for upregulation of XT-I and sustained GAG synthesis prior to the development of cartilage lesions during the pathogenic process of OA.
Conflict of interest statement
Figures







References
-
- Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nature Reviews Rheumatology. 2011;7:50–56. - PubMed
-
- Mankin HJ, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52:424–434. - PubMed
-
- Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. - PubMed
-
- Kapoor M, Johanne Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. osteoarthritis. Nature Reviews Rheumatology. 2011;7:33–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

