The regulatory repertoire of Pseudomonas aeruginosa AmpC ß-lactamase regulator AmpR includes virulence genes
- PMID: 22479525
- PMCID: PMC3315558
- DOI: 10.1371/journal.pone.0034067
The regulatory repertoire of Pseudomonas aeruginosa AmpC ß-lactamase regulator AmpR includes virulence genes
Abstract
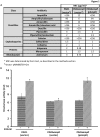
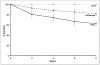
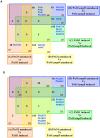
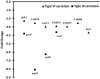
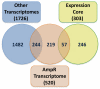
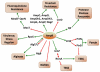
In Enterobacteriaceae, the transcriptional regulator AmpR, a member of the LysR family, regulates the expression of a chromosomal β-lactamase AmpC. The regulatory repertoire of AmpR is broader in Pseudomonas aeruginosa, an opportunistic pathogen responsible for numerous acute and chronic infections including cystic fibrosis. In addition to regulating ampC, P. aeruginosa AmpR regulates the sigma factor AlgT/U and production of some quorum sensing (QS)-regulated virulence factors. In order to better understand the ampR regulon, we compared the transcriptional profile generated using DNA microarrays of the prototypic P. aeruginosa PAO1 strain with its isogenic ampR deletion mutant, PAOΔampR. Transcriptome analysis demonstrates that the AmpR regulon is much more extensive than previously thought, with the deletion of ampR influencing the differential expression of over 500 genes. In addition to regulating resistance to β-lactam antibiotics via AmpC, AmpR also regulates non-β-lactam antibiotic resistance by modulating the MexEF-OprN efflux pump. Other virulence mechanisms including biofilm formation and QS-regulated acute virulence factors are AmpR-regulated. Real-time PCR and phenotypic assays confirmed the microarray data. Further, using a Caenorhabditis elegans model, we demonstrate that a functional AmpR is required for P. aeruginosa pathogenicity. AmpR, a member of the core genome, also regulates genes in the regions of genome plasticity that are acquired by horizontal gene transfer. Further, we show differential regulation of other transcriptional regulators and sigma factors by AmpR, accounting for the extensive AmpR regulon. The data demonstrates that AmpR functions as a global regulator in P. aeruginosa and is a positive regulator of acute virulence while negatively regulating biofilm formation, a chronic infection phenotype. Unraveling this complex regulatory circuit will provide a better understanding of the bacterial response to antibiotics and how the organism coordinately regulates a myriad of virulence factors in response to antibiotic exposure.
Conflict of interest statement
Figures



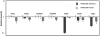


References
-
- Bouza E, Burillo A, Munoz P. Catheter-related infections: diagnosis and intravascular treatment. Clin Microbiol Infect. 2002;8:265–274. - PubMed
-
- Gallagher PG, Watanakunakorn C. Pseudomonas bacteremia in a community teaching hospital, 1980–1984. Rev Infect Dis. 1989;11:846–852. - PubMed
-
- Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33:947–953. - PubMed
-
- Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002;3:128–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

