EGF-induced EMT and invasiveness in serous borderline ovarian tumor cells: a possible step in the transition to low-grade serous carcinoma cells?
- PMID: 22479527
- PMCID: PMC3316602
- DOI: 10.1371/journal.pone.0034071
EGF-induced EMT and invasiveness in serous borderline ovarian tumor cells: a possible step in the transition to low-grade serous carcinoma cells?
Abstract
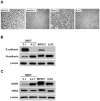
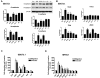
In high-grade ovarian cancer cultures, it has been shown that epidermal growth factor (EGF) induces cell invasion by activating an epithelial-mesenchymal transition (EMT). However, the effect of EGF on serous borderline ovarian tumors (SBOT) and low-grade serous carcinomas (LGC) cell invasion remains unknown. Here, we show that EGF receptor (EGFR) was expressed, that EGF treatment increased cell migration and invasion in two cultured SBOT cell lines, SBOT3.1 and SV40 large T antigen-infected SBOT cells (SBOT4-LT), and in two cultured LGC cell lines, MPSC1 and SV40 LT/ST-immortalized LGC cells (ILGC). However, EGF induced down-regulation of E-cadherin and concurrent up-regulation of N-cadherin in SBOT cells but not in LGC cells. In SBOT cells, the expression of the transcriptional repressors of E-cadherin, Snail, Slug and ZEB1 were increased by EGF treatment. Treatment with EGF led to the activation of the downstream ERK1/2 and PI3K/Akt. The MEK1 inhibitor PD98059 diminished the EGF-induced cadherin switch and the up-regulation of Snail, Slug and ZEB1 and the EGF-mediated increase in SBOT cell migration and invasion. The PI3K inhibitor LY294002 had similar effects, but it could not block the EGF-induced up-regulation of N-cadherin and ZEB1. This study demonstrates that EGF induces SBOT cell migration and invasion by activating EMT, which involves the activation of the ERK1/2 and PI3K/Akt pathways and, subsequently, Snail, Slug and ZEB1 expression. Moreover, our results suggest that there are EMT-independent mechanisms that mediate the EGF-induced LGC cell migration and invasion.
Conflict of interest statement
Figures



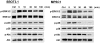
References
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
-
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. - PubMed
-
- Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101:816–829. - PubMed
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

