Exploring the switchgrass transcriptome using second-generation sequencing technology
- PMID: 22479570
- PMCID: PMC3315583
- DOI: 10.1371/journal.pone.0034225
Exploring the switchgrass transcriptome using second-generation sequencing technology
Abstract
Background: Switchgrass (Panicum virgatum L.) is a C4 perennial grass and widely popular as an important bioenergy crop. To accelerate the pace of developing high yielding switchgrass cultivars adapted to diverse environmental niches, the generation of genomic resources for this plant is necessary. The large genome size and polyploid nature of switchgrass makes whole genome sequencing a daunting task even with current technologies. Exploring the transcriptional landscape using next generation sequencing technologies provides a viable alternative to whole genome sequencing in switchgrass.
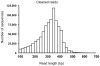
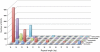
Principal findings: Switchgrass cDNA libraries from germinating seedlings, emerging tillers, flowers, and dormant seeds were sequenced using Roche 454 GS-FLX Titanium technology, generating 980,000 reads with an average read length of 367 bp. De novo assembly generated 243,600 contigs with an average length of 535 bp. Using the foxtail millet genome as a reference greatly improved the assembly and annotation of switchgrass ESTs. Comparative analysis of the 454-derived switchgrass EST reads with other sequenced monocots including Brachypodium, sorghum, rice and maize indicated a 70-80% overlap. RPKM analysis demonstrated unique transcriptional signatures of the four tissues analyzed in this study. More than 24,000 ESTs were identified in the dormant seed library. In silico analysis indicated that there are more than 2000 EST-SSRs in this collection. Expression of several orphan ESTs was confirmed by RT-PCR.
Significance: We estimate that about 90% of the switchgrass gene space has been covered in this analysis. This study nearly doubles the amount of EST information for switchgrass currently in the public domain. The celerity and economical nature of second-generation sequencing technologies provide an in-depth view of the gene space of complex genomes like switchgrass. Sequence analysis of closely related members of the NAD(+)-malic enzyme type C4 grasses such as the model system Setaria viridis can serve as a viable proxy for the switchgrass genome.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

