Heterogeneity of inflammatory and cytokine networks in chronic plaque psoriasis
- PMID: 22479649
- PMCID: PMC3315545
- DOI: 10.1371/journal.pone.0034594
Heterogeneity of inflammatory and cytokine networks in chronic plaque psoriasis
Abstract
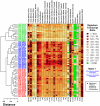
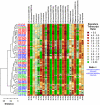
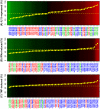
The clinical features of psoriasis, characterized by sharply demarcated scaly erythematous plaques, are typically so distinctive that a diagnosis can easily be made on these grounds alone. However, there is great variability in treatment response between individual patients, and this may reflect heterogeneity of inflammatory networks driving the disease. In this study, whole-genome transcriptional profiling was used to characterize inflammatory and cytokine networks in 62 lesional skin samples obtained from patients with stable chronic plaque psoriasis. We were able to stratify lesions according to their inflammatory gene expression signatures, identifying those associated with strong (37% of patients), moderate (39%) and weak inflammatory infiltrates (24%). Additionally, we identified differences in cytokine signatures with heightened cytokine-response patterns in one sub-group of lesions (IL-13-strong; 50%) and attenuation of these patterns in a second sub-group (IL-13-weak; 50%). These sub-groups correlated with the composition of the inflammatory infiltrate, but were only weakly associated with increased risk allele frequency at some psoriasis susceptibility loci (e.g., REL, TRAF3IP2 and NOS2). Our findings highlight variable points in the inflammatory and cytokine networks known to drive chronic plaque psoriasis. Such heterogeneous aspects may shape clinical course and treatment responses, and can provide avenues for development of personalized treatments.
Conflict of interest statement
Figures

References
-
- Gudjonsson JE, Elder JT. Psoriasis. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AM, editors. Fitzpatrick's Dermatology in General Medicine. New York: McGraw-Hill; 2007. pp. 169–194.
-
- Balato N, Di Costanzo L, Balato A. Differential diagnosis of psoriasis. J Rheumatol. 2009;83:24–25. - PubMed
-
- Wendling D, Balblanc JC, Briançon D, Brousse A, Lohse A, et al. Onset or exacerbation of cutaneous psoriasis during TNFalpha antagonist therapy. Joint Bone Spine. 2008;75:315–318. - PubMed
-
- Viguier M, Richette P, Bachelez H, Wendling D, Aubin F. Paradoxical adverse effects of anti-TNF-alpha treatment: onset or exacerbation of cutaneous disorders. Expert Rev Clin Immunol. 2009;5:421–431. - PubMed
-
- Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J Am Acad Dermatol. 2012;66:e33–e45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

