A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection
- PMID: 22479663
- PMCID: PMC3313920
- DOI: 10.1371/journal.pntd.0001591
A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection
Abstract
Schistosomiasis, a neglected tropical disease, owes its continued success to freshwater snails that support production of prolific numbers of human-infective cercariae. Encounters between schistosomes and snails do not always result in the snail becoming infected, in part because snails can mount immune responses that prevent schistosome development. Fibrinogen-related protein 3 (FREP3) has been previously associated with snail defense against digenetic trematode infection. It is a member of a large family of immune molecules with a unique structure consisting of one or two immunoglobulin superfamily domains connected to a fibrinogen domain; to date fibrinogen containing proteins with this arrangement are found only in gastropod molluscs. Furthermore, specific gastropod FREPs have been shown to undergo somatic diversification. Here we demonstrate that siRNA mediated knockdown of FREP3 results in a phenotypic loss of resistance to Schistosoma mansoni infection in 15 of 70 (21.4%) snails of the resistant BS-90 strain of Biomphalaria glabrata. In contrast, none of the 64 control BS-90 snails receiving a GFP siRNA construct and then exposed to S. mansoni became infected. Furthermore, resistance to S. mansoni was overcome in 22 of 48 snails (46%) by pre-exposure to another digenetic trematode, Echinostoma paraensei. Loss of resistance in this case was shown by microarray analysis to be associated with strong down-regulation of FREP3, and other candidate immune molecules. Although many factors are certainly involved in snail defense from trematode infection, this study identifies for the first time the involvement of a specific snail gene, FREP3, in the phenotype of resistance to the medically important parasite, S. mansoni. The results have implications for revealing the underlying mechanisms involved in dictating the range of snail strains used by S. mansoni, and, more generally, for better understanding the phenomena of host specificity and host switching. It also highlights the role of a diversified invertebrate immune molecule in defense against a human pathogen. It suggests new lines of investigation for understanding how susceptibility of snails in areas endemic for S. mansoni could be manipulated and diminished.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



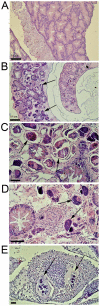
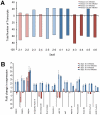
References
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet. 2006;6:411–425. - PubMed
-
- Ward RD, Lewis FA, Yoshino TP, Dunn TS. Schistosoma mansoni: relationship between cercarial production levels and snail host susceptibility. Exp Parasitol. 1988;66:78–85. - PubMed
-
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitol. 2009;136:1719–1730. - PubMed
-
- Mone Y, Ribou AC, Cosseau C, Duval D, Theron A, et al. An example of molecular co-evolution: Reactive oxygen species (ROS) and ROS scavenger levels in Schistosoma mansoni/Biomphalaria glabrata interactions. Int J Parasitol. 2011;41:721–730. - PubMed
-
- Goodall CP, Bender RC, Brooks JK, Bayne CJ. Biomphalaria glabrata cytosolic copper/zinc superoxide dismutase (SOD1) gene: association of SOD1 alleles with resistance/susceptibility to Schistosoma mansoni. Mol Biochem Parasitol. 2006;147:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

