Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis
- PMID: 22480579
- PMCID: PMC3348331
- DOI: 10.1016/j.tcb.2012.02.007
Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis
Abstract
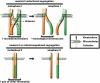
The ability to reproduce relies in most eukaryotes on specialized cells called gametes. Gametes are formed by the process of meiosis in which, after a single round of replication, two successive cell divisions reduce the ploidy of the genome. Fusion of gametes at fertilization reconstitutes diploidy. In most animal species, chromosome segregation during female meiosis occurs on spindles assembled in the absence of the major microtubule-organizing center, the centrosome. In mammals, oocyte meiosis is error prone and underlies most birth aneuploidies. Here, we review recent work on acentrosomal spindle formation and chromosome alignment/separation during oocyte meiosis in different animal models.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. - PubMed
-
- Manandhar G, et al. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. - PubMed
-
- Szöllösi D, et al. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972;11:521–541. - PubMed
-
- Dumont J, Brunet S. Meiotic Spindle Assembly and Chromosome Segregation in Oocytes. In: Verlhac MH, Villeneuve AM, editors. Oogenesis: The Universal Process. Wiley-Blackwell; 2010. pp. 269–290.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

