Genetics of atherosclerosis
- PMID: 22480919
- PMCID: PMC3362664
- DOI: 10.1016/j.tig.2012.03.001
Genetics of atherosclerosis
Abstract
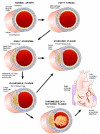
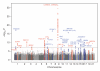
Genome-wide association studies (GWAS) from the past several years have provided the first unbiased evidence of the genes contributing to common cardiovascular disease traits in European and some Asian populations. The results not only confirmed the importance of prior knowledge, such as the central role of lipoproteins, but also revealed that there is still much to learn about the underlying mechanisms of this disease, as most of the associated genes do not appear to be involved in pathways previously connected to atherosclerosis. In this review, I focus on the common forms of the disease and look at both human and animal model studies. I summarize what was known before GWAS, highlight how the field has been changed by GWAS, and discuss future considerations, such as the limitations of GWAS and strategies that may lead to a more complete, mechanistic understanding of atherosclerosis.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
- R01 HL094322/HL/NHLBI NIH HHS/United States
- DP3 D094311/DP/NCCDPHP CDC HHS/United States
- P01 HL305685/HL/NHLBI NIH HHS/United States
- DP3 DK094311/DK/NIDDK NIH HHS/United States
- P01 HL028481/HL/NHLBI NIH HHS/United States
- R01 HL0943221/HL/NHLBI NIH HHS/United States
- R21 HL110667-01/HL/NHLBI NIH HHS/United States
- 1R01 GM0956561/GM/NIGMS NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
- R01 GM095656/GM/NIGMS NIH HHS/United States
- R21 HL110667/HL/NHLBI NIH HHS/United States
- P01 HL28481/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

