Immunization strategies against henipaviruses
- PMID: 22481140
- PMCID: PMC4465348
- DOI: 10.1007/82_2012_213
Immunization strategies against henipaviruses
Abstract
Hendra virus and Nipah virus are recently discovered and closely related emerging viruses that now comprise the genus henipavirus within the sub-family Paramyxoviridae and are distinguished by their broad species tropism and in addition to bats can infect and cause fatal disease in a wide variety of mammalian hosts including humans. The high mortality associated with human and animal henipavirus infections has highlighted the importance and necessity of developing effective immunization strategies. The development of suitable animal models of henipavirus infection and pathogenesis has been critical for testing the efficacy of potential therapeutic approaches. Several henipavirus challenge models have been used and recent successes in both active and passive immunization strategies against henipaviruses have been reported which have all targeted the viral envelope glycoproteins.
Conflict of interest statement
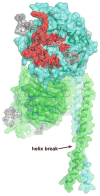
Figures


References
-
- Anonymous. Hendra virus, human, equine. Australia (04), Queensland: Pro-MED-mail, International Society for Infectious Diseases; 2008. Jul 25, archive no. 20080725.2260. Available at www.promedmail.org.
-
- Anonymous. Human, Equine. Australia (04), Queensland Fatal: Pro-MED-mail, International Society for Infectious Diseases; 2009. Sep 3, archive no. 20090903.3098. Available at www.promedmail.org.
-
- Anonymous. Hendra Virus, Equine. Australia (28), Queensland, New South Wales: Pro-MED-mail International Society for Infectious Diseases; 2011. Oct 12, archive no. 20111013.3061. Available at www.promedmail.org.
-
- Anonymous. Hendra virus, equine. Australia, Queensland: Pro-MED-mail International Society for Infectious Diseases; 2012a. Jan 6, archive no. 20120106.1001359. Available at www.promedmail.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

