Histopathology of incidental findings in beagles used in toxicity studies
- PMID: 22481862
- PMCID: PMC3320160
- DOI: 10.1293/tox.25.103
Histopathology of incidental findings in beagles used in toxicity studies
Abstract
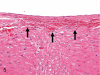
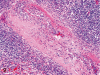
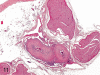
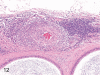
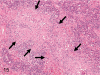
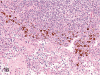
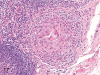
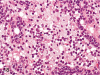
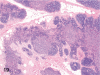
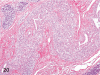
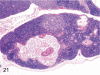
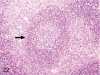
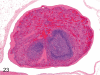
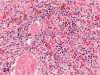
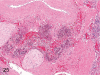
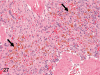
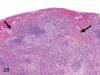
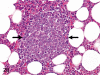
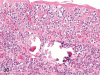
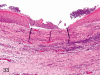
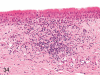
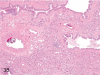
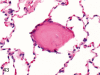
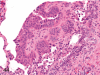
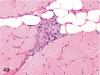
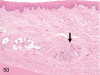
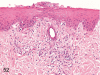
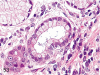
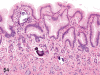
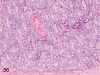
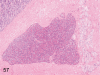
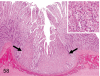
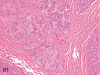
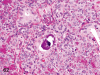
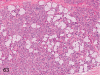
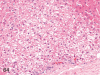
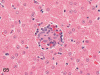
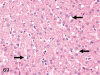
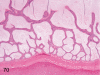
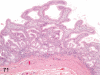
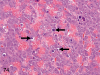
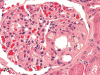
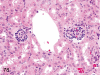
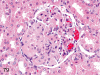
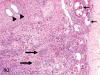
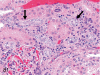
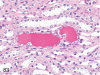
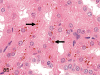
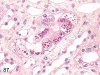
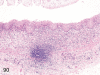
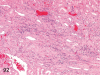
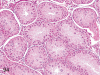
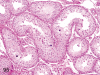
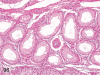
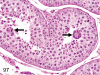
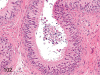
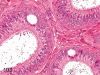
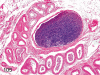
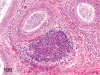
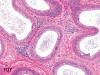
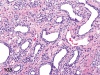
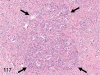
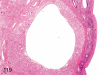
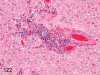
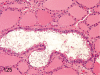
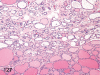
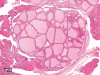
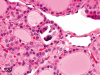
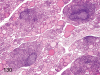
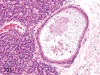
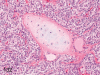
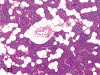
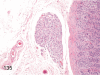
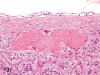
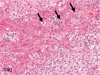
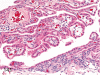
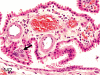
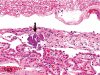
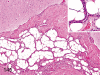
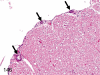
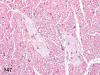
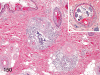
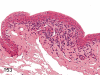
The purpose of our publication is to widely communicate the pictures of spontaneous findings occurring in beagles. Spontaneous arteritis occurs commonly in beagles. Frequent sites of arteritis are the heart, spleen, pancreas, epididymis and spinal cord. Morphological similarities between spontaneous and drug-induced arterial lesions may cause confusion when evaluating vascular toxicity of chemicals such as vasodilating agents. Focal and minimal inflammatory lesions are occasionally seen in the lung and may be associated with aspiration of food particles or of unknown causes. A cystic change with copious mucin production occurs occasionally in the mucosal epithelium of the gall bladder. Nesidioblastosis is seen rarely in the pancreas of beagles. C-cell complex and lymphocytic thyroiditis are common thyroid lesions. Spontaneous focal hypospermatogenesis and lobular Sertoli-cell-only seminiferous tubules occurring frequently in beagles must be distinguished from drug-induced damage of the seminiferous tubules in toxicity studies. The morphological differences of the female genital system in each cycle need to be understood; therefore, we present the normal features of the cyclic changes of the female genital organs. Further, we provide more information on spontaneous findings in beagles for exact diagnoses in toxicity studies.
Keywords: background; beagle; histopathology; incidental; spontaneous.
Figures

















































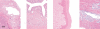




















References
-
- Jones TC, Hunt RD, King NW. Veterinary Pathology. 6th ed. Lippincott Williams & Wilkins, Baltimore. 1997
-
- Maxie MG. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed. Elsevier, Philadelphia. 2007
-
- Mohr U, Carlton WW, Dungworth DL, Benjamin SA, Capen CC, Hahn FF. Pathobiology of the Aging Dog. Iowa State University Press, Ames. 2001
-
- Japanese Society of Veterinary Pathology. Colour Atlas of Animal Pathology. Bun-eido Publishing, Tokyo. 2007. (in Japanese).
-
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Dignosis. Blackwell Science, Oxford. 2005
LinkOut - more resources
Full Text Sources
