Pixel-based machine learning in medical imaging
- PMID: 22481907
- PMCID: PMC3299341
- DOI: 10.1155/2012/792079
Pixel-based machine learning in medical imaging
Abstract
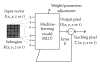
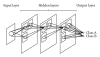
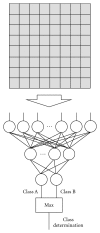
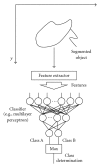
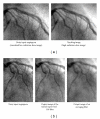
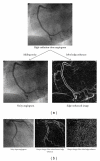
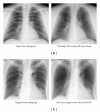
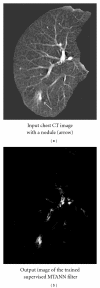
Machine learning (ML) plays an important role in the medical imaging field, including medical image analysis and computer-aided diagnosis, because objects such as lesions and organs may not be represented accurately by a simple equation; thus, medical pattern recognition essentially require "learning from examples." One of the most popular uses of ML is classification of objects such as lesions into certain classes (e.g., abnormal or normal, or lesions or nonlesions) based on input features (e.g., contrast and circularity) obtained from segmented object candidates. Recently, pixel/voxel-based ML (PML) emerged in medical image processing/analysis, which use pixel/voxel values in images directly instead of features calculated from segmented objects as input information; thus, feature calculation or segmentation is not required. Because the PML can avoid errors caused by inaccurate feature calculation and segmentation which often occur for subtle or complex objects, the performance of the PML can potentially be higher for such objects than that of common classifiers (i.e., feature-based MLs). In this paper, PMLs are surveyed to make clear (a) classes of PMLs, (b) similarities and differences within (among) different PMLs and those between PMLs and feature-based MLs, (c) advantages and limitations of PMLs, and (d) their applications in medical imaging.
Figures





References
-
- Giger ML, Suzuki K. Computer-aided diagnosis (CAD) In: Feng DD, editor. Biomedical Information Technology. New York, NY, USA: Academic Press; 2007. pp. 359–374.
-
- Doi K. Current status and future potential of computer-aided diagnosis in medical imaging. British Journal of Radiology. 2005;78(supplement 1):S3–S19. - PubMed
-
- Li F, Sone S, Abe H, MacMahon H, Armato SG, III, Doi K. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology. 2002;225(3):673–683. - PubMed
-
- Lostumbo A, Wanamaker C, Tsai J, Suzuki K, Dachman AH. Comparison of 2D and 3D views for evaluation of flat lesions in CT colonography. Academic Radiology. 2010;17(1):39–47. - PubMed
-
- Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. Journal of the American Medical Association. 2008;299(9):1027–1035. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

